Dräger Medical AG & Co. KGaA 6173.3
All rights reserved. Copyright reserved.
_ _Printed on_18.05.05_F61733XXT01.fm
55
Function description Babylog 8000
11.3 O
2
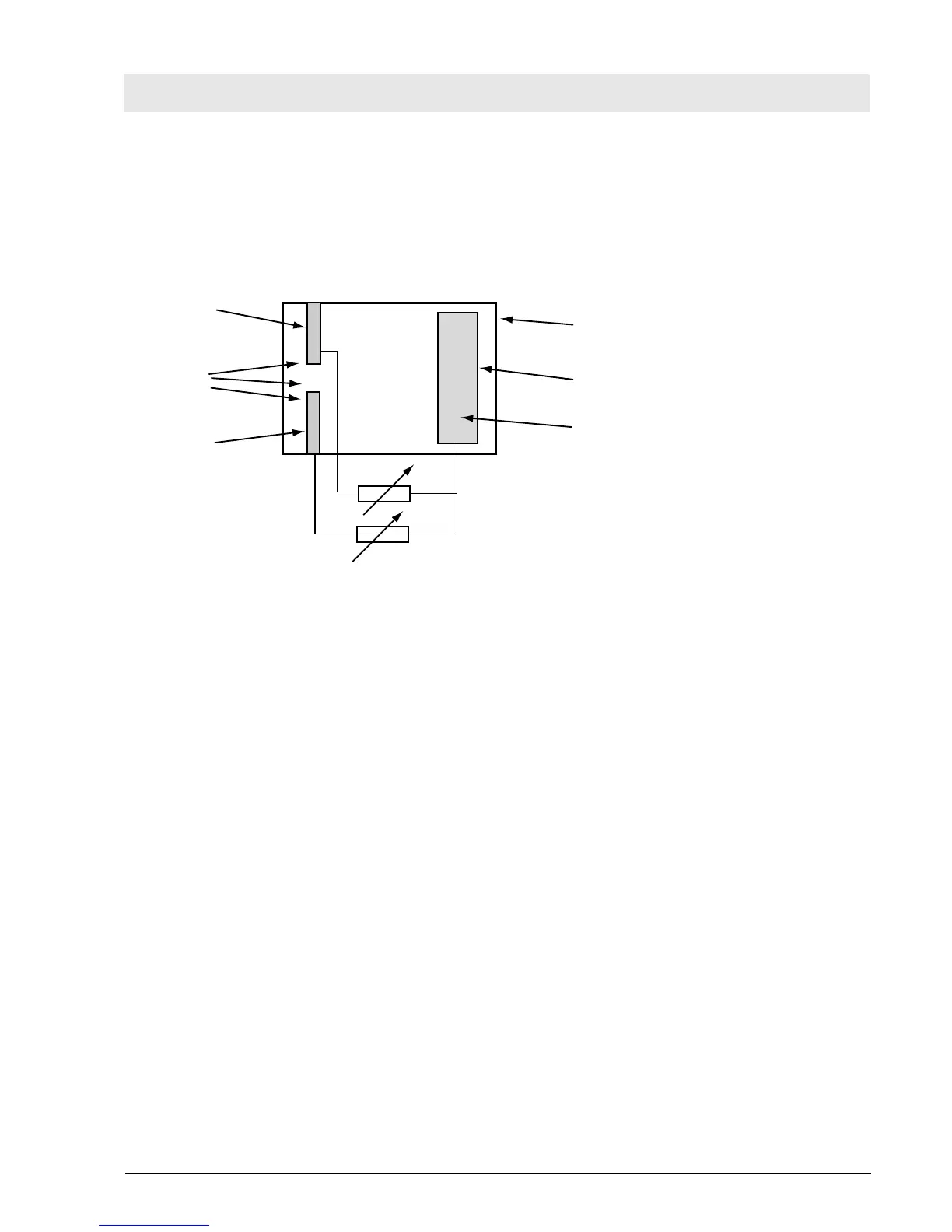
Sensor
The O
2
sensor is a double cell which functions according to the fuel-cell principle, that is, it is an
electrochemical cell which generates a voltage by means of an ion current. The cell consists of a capsule
which incorporates electrolytes, lead anodes and two gold cathodes with Teflon diaphragm.
Fig. 30: O
2
sensor cell
The oxygen to be measured diffuses through the Teflon diaphragm, undergoes a chemical reaction at the
gold cathode and produces lead oxide and water at the lead anode. During this chemical process an
electric voltage is generated which is proportional to the oxygen partial pressure. The gold cathodes are
negative, the lead anode is positive. The internal resistance is determined by the surface of the
electrodes, the oxygen diffusion velocity, and the distances. Under normal condition, the internal
resistance is 700 ohms.
Like most chemical processes, this process is also temperature-dependent. Therefore, compensation
resistors are connected in parallel to the sensor. These resistors correct the measuring voltage in relation
to the internal resistance. Two voltages are generated which are identical within specified tolerances. The
voltages are compared to each other. The two gold cathodes are arranged separately. The two voltages
do not influence each other. If one gold cathode fails, the other carries out the measurement. In this case,
the electronics would report a malfunction.
cathode 1
cathode 2
O2
Plastic housing
Alkali-Elektrolyt
anode
-

 Loading...
Loading...