Care and Maintenance
4-32 Ultrasound System – Common Service Information
Direction 5444964-100 English
Rev. 5
Lead to lead leakage test record (continued)
NOTE: Not all test procedures are applicable to all areas of the world.
Reversed Polarity testing content satisfies regions following IEC
62353:2007 and IEC 60601-1:2005.
NOTE: Values in italics font are given as examples only.
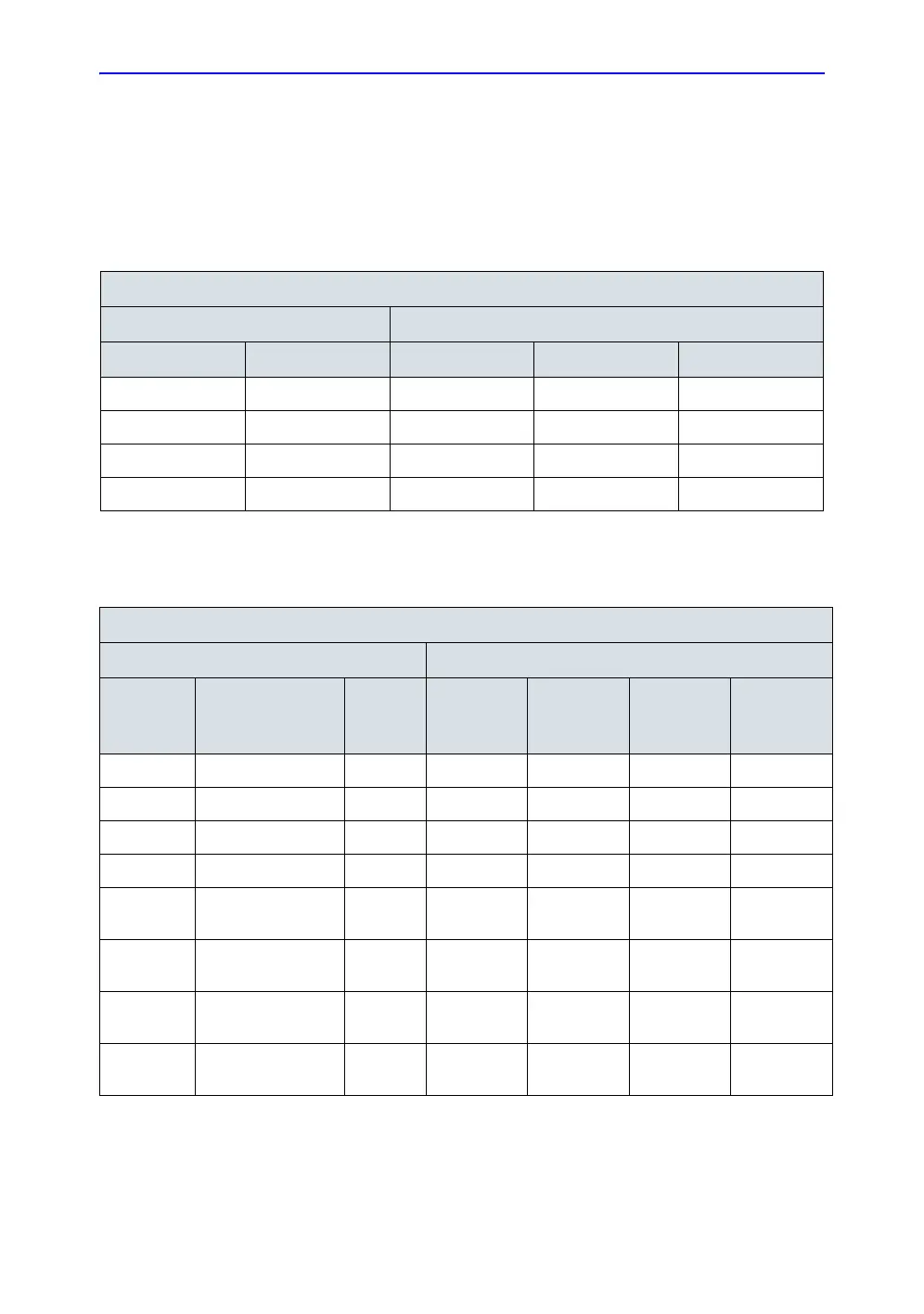
Table 4-12: Typical data format for recording patient lead to lead leakage
Unit under test____________________________________ Date of test:_____________
Test Conditions Patient Lead or Combination Measured
System Power Grounding/PE RA to LA LA to LL LL to RA
Off closed
Off open
On (Transmit) closed
On (Transmit) open
Table 4-13: Typical data format for recording patient lead to lead leakage
Unit under test____________________________________ Date of test:_____________
Test Conditions Patient Lead or Combination Measured
System
Power Grounding/PE
Limit
µA RA to LA LA to LL LL to RA
(RA+LA+
LL) to
GRND
off closed 10
off open 50
on closed 10
on open 50
off closed (reversed
polarity)
10
off open (reversed
polarity)
50
on closed (reversed
polarity)
10
on open (reversed
polarity)
50

 Loading...
Loading...