IM-NE-C101-D_rev 05_05_17_updated.pdf
- This device (compressor, nebulizer kit, air tube, adapter, mouthpiece, nosepiece,
adult and child mask) complies with the requirements of the European Standard
EN ISO 27427:2019 Anaesthetic and respiratory equipment - Nebulizing systems and
components.
- The nebulizer performance may vary with drugs such as suspensions or high viscosity
solutions. See drug supplier’s data sheet for further details.
- The particle size distribution and aerosol output may vary by combination of product,
medication and ambient conditions such as temperature, humidity and atmospheric
pressure.
- The disclosures for nebulizer performance are based upon testing that utilizes adult
ventilatory patterns and are likely to be different from those stated for paediatric or
infant populations.
Troubleshooting
In case of any of the below problems occur during use, first check that no other electrical
device is within 30 cm. If the problem persists, please refer to the below.
The device does not switch on
• Check that the power plug is properly fitted to the power outlet.
• Make sure the power switch is in the on position ( ).
The device switches on but does not nebulize
• Make sure the vaporizer head is fitted in the nebulizer kit.
• Make sure the air tube is not squashed or crooked.
• Check the air filter for blockage and dirt. Replace if necessary.
• Check that sufficient amount of medication has been put into the nebulizer kit.
The device suddenly stops working during operation.
• The thermal cut-out has shut the device down for one of the following reasons:
- the device was working in an environment with temperatures higher than 40°C;
- the ventilation slots were covered.
Do not attempt to repair the device. Do not open and/or tamper with the device. No parts
of the device are user serviceable. Return the device to an authorized OMRON retail
outlet or distributor.
Please report to the manufacturer and the competent authority of the Member State in
which you are established about any serious incident that has occurred in relation to this
device.
Warranty
Thank you for buying an OMRON product. This product is constructed of high quality
materials and great care has been taken in its manufacturing. It is designed to give you
the highest level of comfort, provided it is properly operated and maintained as described
in the instruction manual. This product does not require maintenance apart from regular
cleaning as described in the ‘Cleaning and disinfecting‘ section. This product is guaranteed
by OMRON for a period of 3 years after the date of purchase.
The proper construction, workmanship and materials of this product is guaranteed by
OMRON. During this period of guarantee OMRON will, without charge for labour or parts,
repair or replace the defect product or any defective parts. The guarantee does not cover
any of the following:
a. Transport costs and risks of transport.
b. Costs for repairs and / or defects resulting from repairs done by unauthorized persons.
c. Failure or wear of optional parts or other attachments other than the main device itself,
unless explicitly guaranteed above.
d. Costs arising due to non-acceptance of a claim (those will be charged for).
e. Damages of any kind including personal caused accidentally or from misuse.
Should guarantee service be required please apply to the dealer whom the product was
purchased from or an authorised OMRON distributor. For the address refer to the product
packaging / literature or to your specialised retailer.
Repair or replacement under the guarantee does not give rise to any extension or
renewal of the guarantee period.
The guarantee will be granted only if the complete product is returned together with the
original invoice / cash ticket issued to the consumer by the retailer. OMRON reserves the
right to refuse the guarantee service if any unclear information has been given.
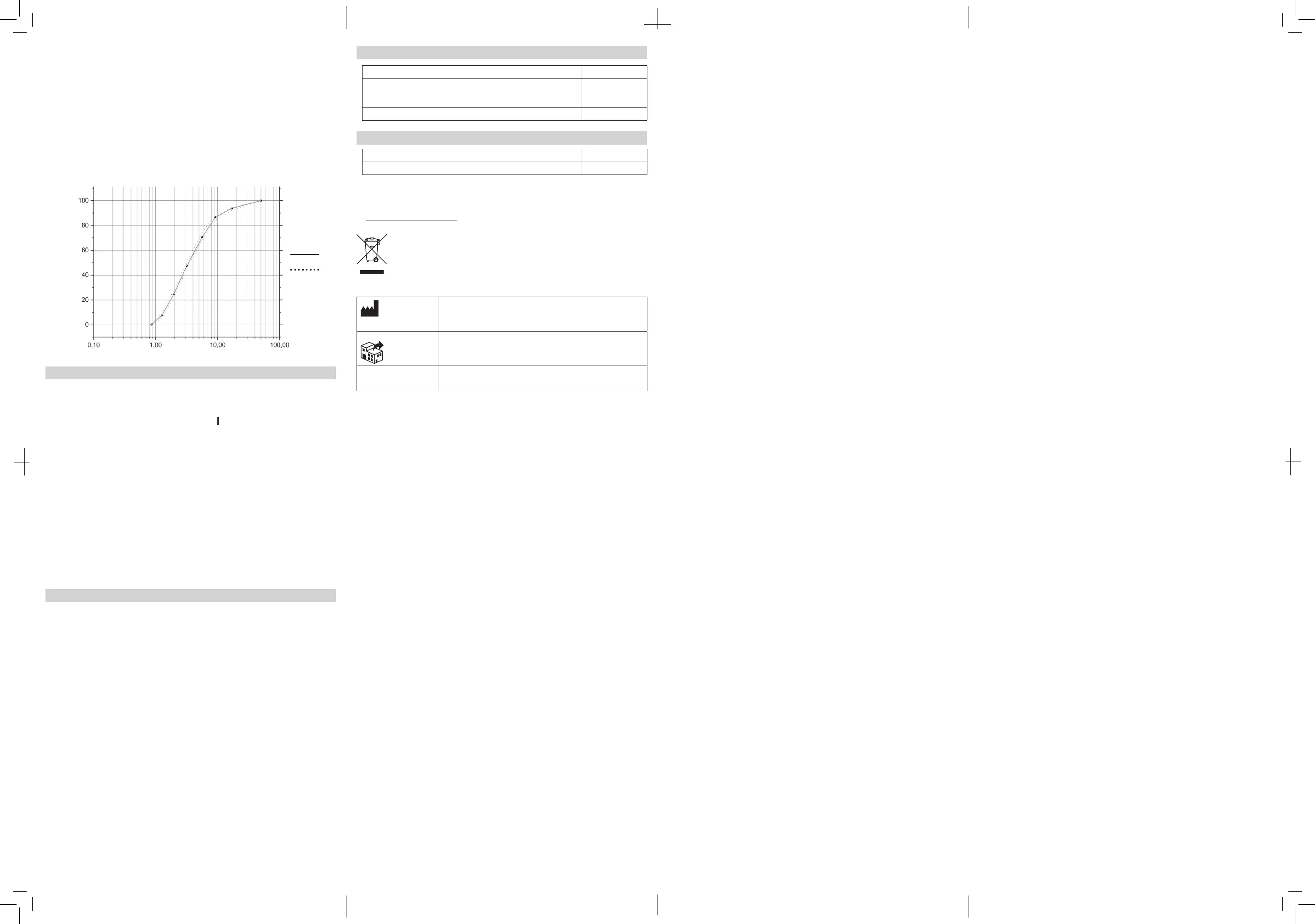
Result of cascade impactor measurements for particle size
Cumulative % particle mass of salbutamol undersize
Particle size Dp (μm)
Cumulative Undersize %
Individual
Mean
Optional Medical Accessories
Product Description Model
Nebulizer Accessory Set
(Contents: Nebulizer Kit, Adapter, Mouthpiece, Nosepiece, Adult
Mask (PVC), Child Mask (PVC), Air Tube, Air Filter)
NEB-ASKIT-11
Nasal Shower NEB6014
Other Optional/Replacement Parts
Product Description Model
Air Filter Set (Contents: 3 pieces) 3AC408
Important information regarding Electro Magnetic Compatibility (EMC)
This device conforms to EN60601-1-2:2015 Electro Magnetic Compatibility (EMC)
standard. Further documentation in accordance with this EMC standard is available at
OMRON HEALTHCARE EUROPE at the address mentioned in this instruction manual or
at www.omron-healthcare.com.
DISPOSAL PROCEDURE (Dir. 2012/19/EU-WEEE)
This product is not to be treated as regular household waste but must be
returned to a collection point for recycling electric and electronic devices.
Further information is available from your municipality, your municipality’s
waste disposal services, or the retailer where you purchased your product.
3A HEALTH CARE S.r.l.
Via Marziale Cerutti, 90F/G
25017 Lonato del Garda (BS),
Italy
Distributor OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, THE NETHERLANDS
www.omron-healthcare.com
Subsidiaries OMRON Niederlassung
OMRON MEDIZINTECHNIK HANDELSGESELLSHAFT mbH
www.omron-healthcare.com/distributors
Made in Italy

 Loading...
Loading...