1
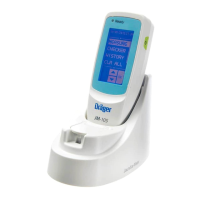
Dräger Jaundice Meter JM-105
Sample Usage Protocol Template
Purpose
This Sample Usage Protocol is provided as a template for creating a facility
or department-specific protocol for using the Dräger Jaundice Meter JM-105.
Description and Intended Use
The Dräger Jaundice Meter JM-105 is intended for use as a screening device
for jaundice in the newborn. The Jaundice Meter JM-105 provides a
transcutaneous measurement of bilirubin in mg/dL or µmol/L, identifying
neonates who require a serum bilirubin measurement.
*1
For the intended use please refer to the instructions for use
Screening for Hyperbilirubinemia
When combined with a systematic assessment of the risk factors for
hyperbilirubinemia, the Dräger Jaundice Meter JM-105 can identify neonates
who are at increased risk for more severe hyperbilirubinemia in the first
week of life, and who might require closer monitoring.
Two protocols are suggested for using the Dräger Jaundice Meter JM-105 as
a screening device for hyperbilirubinemia. One is based on risk factors, the
other is universal screening.












 Loading...
Loading...