LOGIQ e Quick Guide Direction 5130174-100 Rev. 2 23
Using Probes
Probe Cleaning, After Each Use
1. Disconnect probe from ultrasound console and
remove all coupling gel from probe by wiping
with a soft cloth and rinsing with flowing water.
2. Wash the probe with mild soap in lukewarm
water. Scrub the probe as needed using a soft
sponge, gauze, or cloth to remove all visible
residue from the probe surface. Prolonged
soaking or scrubbing with a soft bristle brush
(such as a toothbrush) may be necessary if
material has dried onto the probe surface.
3. Rinse the probe with enough clean potable
water to remove all visible soap residue.
4. Air dry or dry with a soft cloth.
Probe Disinfection, After Each Use
1. Prepare the germicide solution according to the
manufacturer's instructions. Be sure to follow all
precautions for storage, use and disposal.
2. Place the cleaned and dried probe in contact
with the germicide for the time specified by the
germicide manufacturer. High-level disinfection
is recommended for surface probes and is
required for endocavitary and intraoperative
probes (follow the germicide manufacturer's
recommended time).
Probes for neuro surgical intra-operative use
must NOT be sterilized with liquid chemical
sterilants because of the possibility of neuro
toxic residues remaining on the probe.
Neurological procedures must be done with the
use of legally marketed, sterile, pyrogen free
probe sheaths.
3. After removing from the germicide, rinse the
probe following the germicide manufacturer's
rinsing instructions. Flush all visible germicide
residue from the probe and allow to air dry.
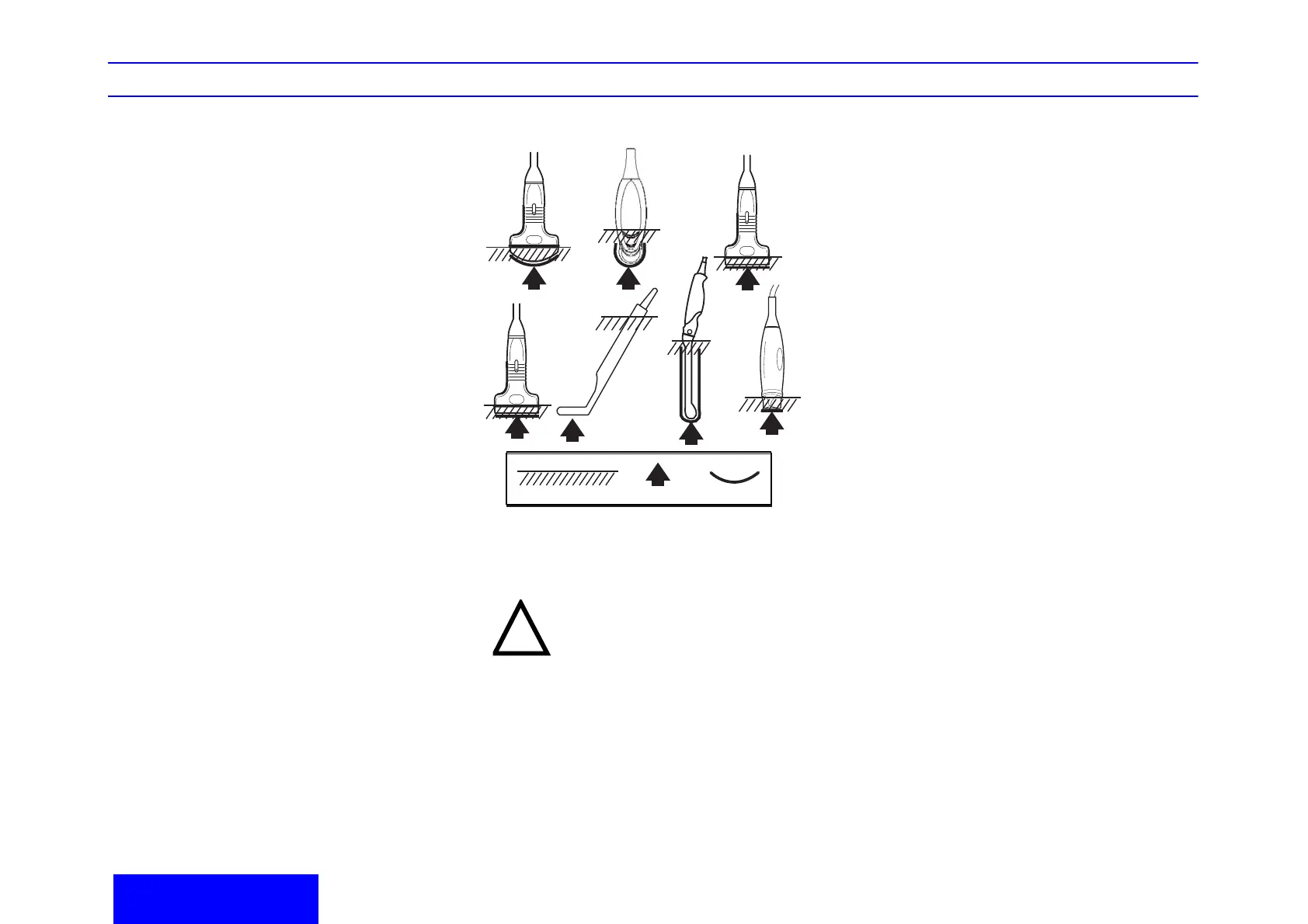
Probe Immersion Levels
1. Fluid Level
2. Aperture
3. Contact face within patient environment
In order for liquid chemical germicides to be effec-
tive, all visible residue must be removed during the
cleaning process. Thoroughly clean the probe, as
described earlier before attempting disinfection.
You MUST disconnect the probe from the LOGIQ e
prior to cleaning/ disinfecting the probe. Failure to
do so could damage the system.
Probe Disinfection Agents
Ultrasound probes can be disinfected using liquid
chemical germicides. The level of disinfection is
directly related to the duration of contact with the
germicide. Increased contact time produces a
higher level of disinfection.
Review the probe care card that is packed with
each probe. The following website contains the
most current and up-to-date recommendations:
http://www.gehealthcare.com/usen/ultrasound/
products/probe_case.html
Cidex Plus has been approved for the all probes
available on LOGIQ e.
Pera Safe high level disinfectant has been
approved for the E8C-RS, 8C-RS and 12L-RS
probes.
T-Spray low level disinfectant has been approved
for the E8C-RS, 8C-RS, 12L-RS, 3S-RS, 8L-RS
probes. And T-Spray II has been approved for all
the probes available on LOGIQ e.
CAUTION
E8C-RS
312
8C-RS
i12L-RS
8L-RS
3S-RS
4C-RS
12L-RS

 Loading...
Loading...