Labels locations
LOGIQ V3/V5/V5 Expert – Basic Service Manual 1-15
5726264-100 English Rev.8
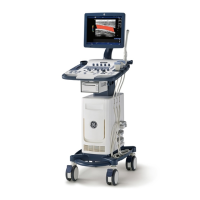
1-5-1 Rating Plate Location(continued)
1. Rating Plate
NOTE: If the new label is needed during the service activities, please
click “Ask an Expert“ to submit the case in the support central:
http://supportcentral.ge.com/products/sup_products.asp?prod_
id=44177.
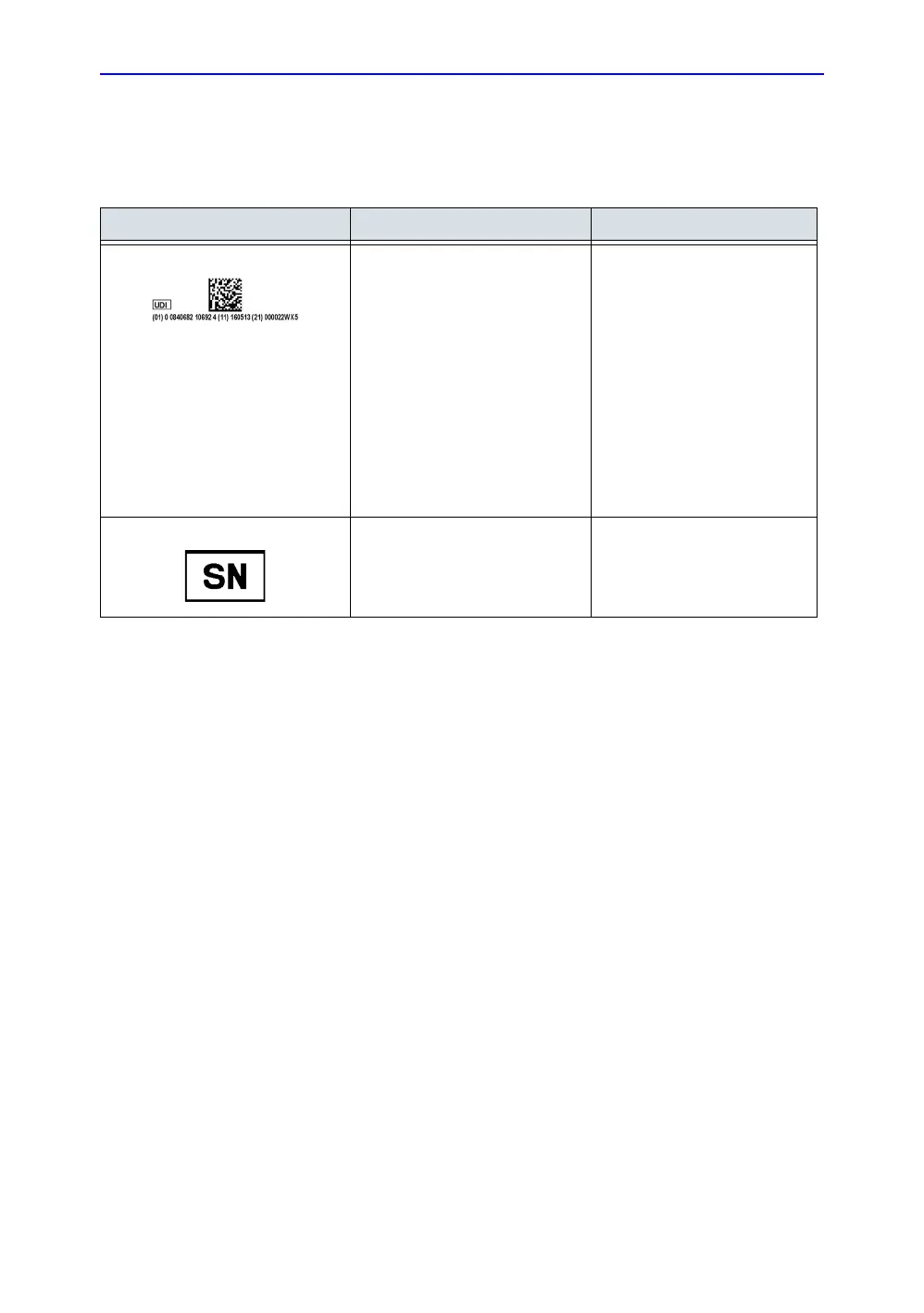
Table 1-5: Label Icons
Label/Icon Purpose/Meaning Location
Every system has a unique
marking for identification, the
Unique Device Identification
(UDI) Label. The UDI label
consists of a series of
alpha-numeric characters and
barcode which uniquely identify
the LOGIQ V3/ V5/V5 Expert
system as a medical device
manufactured by General
Electric. Scan or enter the UDI
information into the patient health
record as required by
country-specific laws.
Rating plate
Serial Number.
Rating plate
 Loading...
Loading...