7 The Freeze-drying process
To understand the freeze-drying process, this section describes the theory for the process in short.
For more detailed information about freeze-drying please consult available literature.
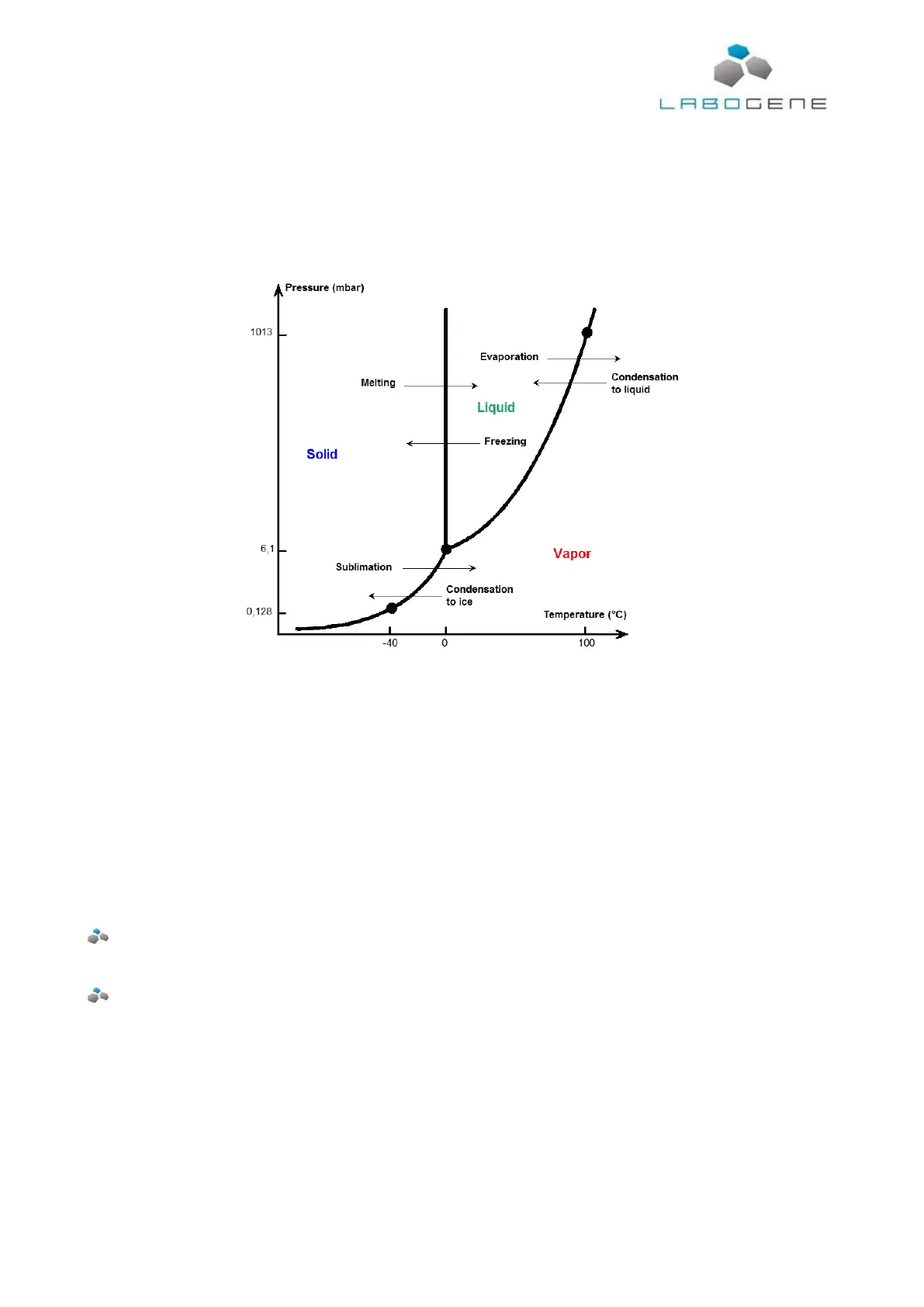
All material in free form will exist in one of three states: Vapour, liquid or solid.
As shown in the diagram for water, the physical state is dependent on the surrounding pressure and
the temperature. The point where all three phases of water co-exist is called the triple point.
Freeze-drying is basically a change in state and properties of the material, (water), that is to be
removed. In principle the process is very simple, but in reality it also can be very complex as the
product can consist of many components, which interact with each other.
Moving from one state to another requires the supply or removal of energy to or from the product.
This energy flux will have an impact on the structure of the material being dried.
Freeze-drying is achieved using equipment that controls temperature and pressure in the
environment of the product and is a 3-step process.
In the first step, the product is frozen solid so that the water present in the material is
converted to ice (pre-freeze).
In the second step, the ice formed in pre-freezing is removed from the product by direct
conversion from solid to vapour. This process is called sublimation. In order to start
removing moisture in the sublimation process, the pressure surrounding the material has to
be brought down to a value below that of the triple point, whilst keeping the temperature of
the product below its freezing point. The condenser surface temperature must also be down
to a temperature lower than the temperature of the material handled. When the product is
heated, it will theoretically place itself somewhere on the transition line between the solid
and vapour states. The heating will initiate sublimation of the moisture from the product to
the surrounding environment. The vapour will move to the condenser surface because of
the lower vapour pressure in the condenser.

 Loading...
Loading...



