2
• This device should be used only by physicians who are trained in standard transcatheter techniques. The physician should
determine which patients are candidates for procedures that use this device.
• Store in a dry place.
Adverse Events
Potential adverse events that may occur during or after a procedure using this delivery system may include, but are not limited
to:
Potential complications specific to balloon sizing include:
• Movement of the balloon toward the mitral valve or right atrium
• Enlargement of the ASD
• Obstruction of venous return from the inferior vena cava
• Difficulty in deflating the balloon
Defect sizing information is provided in table 1, and device dimensions are provided in table 2.
Directions for Use
Recommendations on Guidewire and Balloon Use
The guidewire must remain in place during the procedure. The removal of the guidewire during the procedure can cause kinking
and/or migration of the sizing catheter. The maximum inflation volume of the balloon should not be exceeded to prevent over-
inflation, bursting or detachment of the balloon.
Procedure
Carefully inspect the sterile pouch and verify that it is unopened and undamaged. Gently open the sterile pouch and inspect the
balloon catheter prior to use to verify that it is undamaged. Slowly slide the protective cover off the balloon segment. Avoid acute
bends or kinking of the AMPLATZER™ Sizing Balloon II during removal from the packaging.
The procedure will vary according to the type of occlusion or measurement to be performed. Because of the ultra thin plastic
membrane, dilatation of the entry site is not necessary.For the measurement of secundum atrial septal defects, the following
steps are recommended:
• Arrhythmia • Short-term hemodynamic compromise
• Cardiac obstruction • Stroke
• Death • Thromboembolic event
• Embolization • Valve impingement
• Endocarditis • Vascular thrombosis
• Hematoma • Vessel dissection
• Oversizing of the defect • Vessel perforation
• Pain and tenderness • Vessel spasm
• Sepsis/Infection
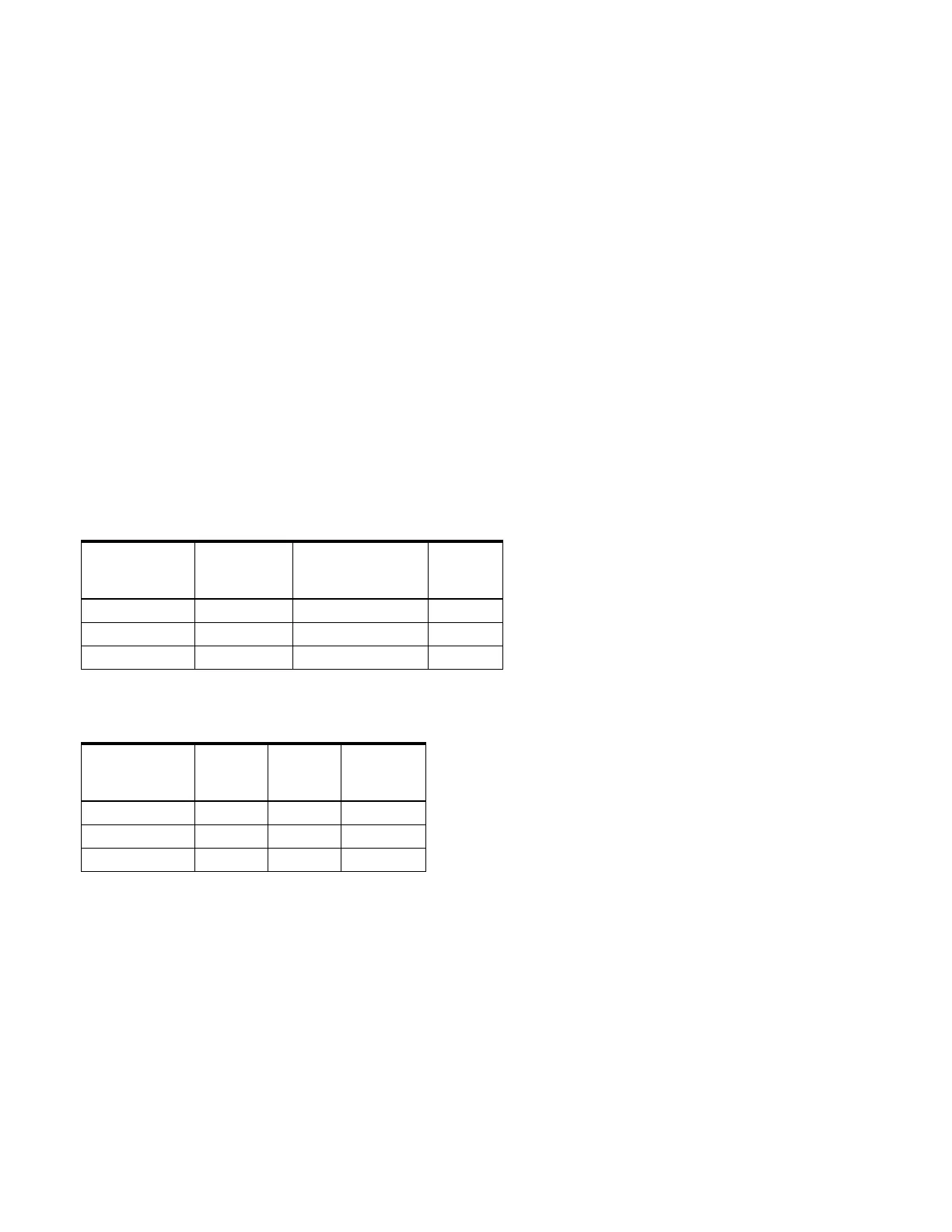
Table 1. Defect sizing parameters
Reference
Number
Maximum
Defect Size
mm
Maximum Inflation
Volume
cc
Balloon
Length
cm/mm
9-SB-018 20 15 3.5/35
9-SB-024 27 30 4.5/45
9-SB-034 40 90 5.5/55
Table 2. Device dimensions
Reference
Number
Shaft
Size
Fr
Usable
Length
cm/mm
Guidewire
in
9-SB-018 6.0 70/700 0.035
9-SB-024 7.0 70/700 0.035
9-SB-034 8.0 70/700 0.035

 Loading...
Loading...