38
3.4 Inspection of accessories
BF-190 Series OPERATION MANUAL
Ch.3
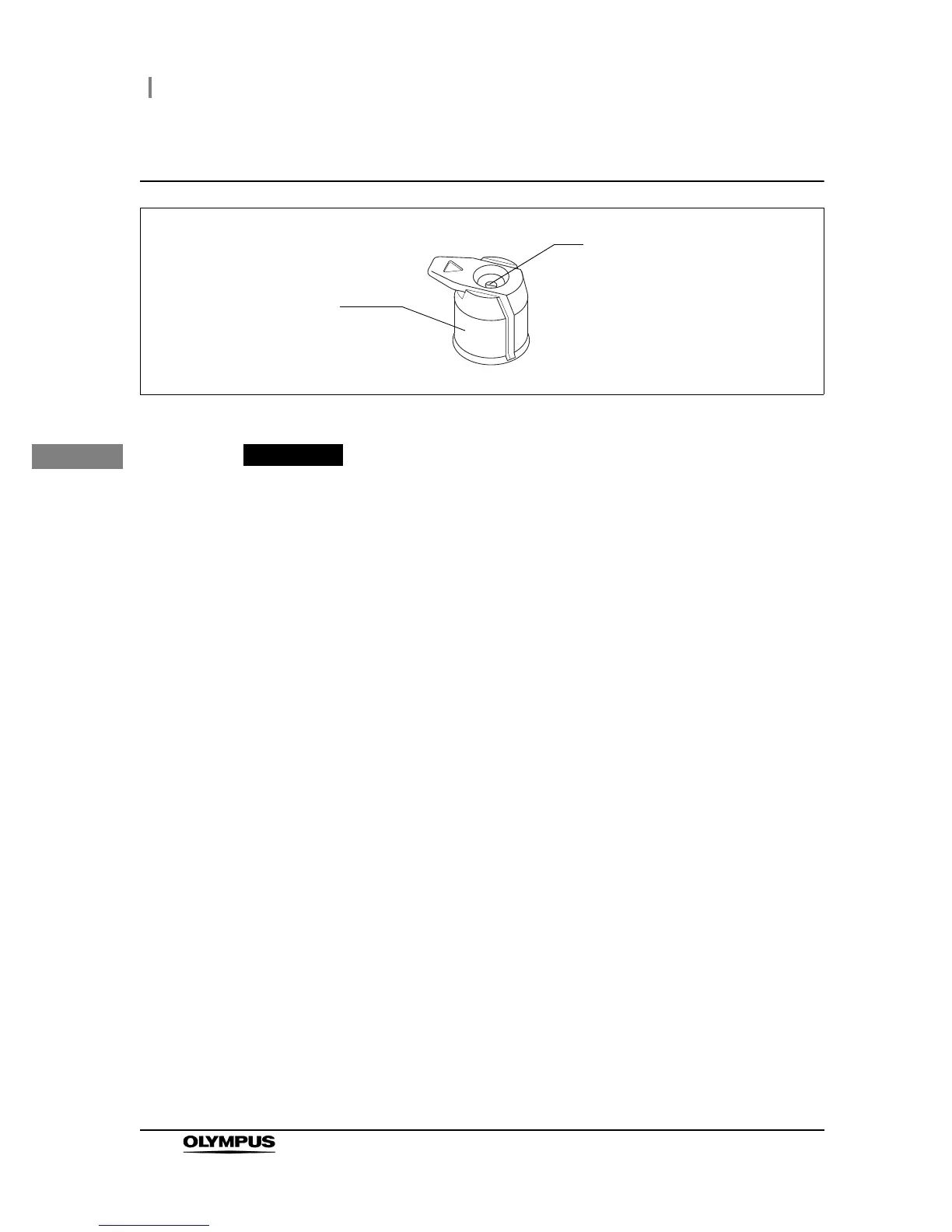
Inspection of the single use biopsy valve (MAJ-210)
Figure 3.14
• Do not use a single use biopsy valve after the expiration date displayed on the
sterile package. Doing so may pose an infection control risk.
• Do not attempt to sterilize the single use biopsy valve. This could pose an infection
control risk or cause equipment damage.
Inspect the single use biopsy valve (MAJ-210) as described in the single use biopsy
valve’s instruction manual.

 Loading...
Loading...











