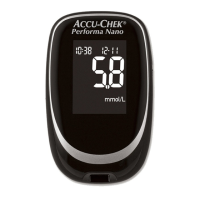
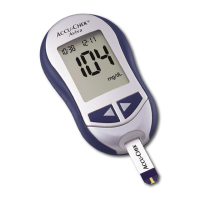
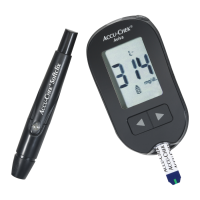
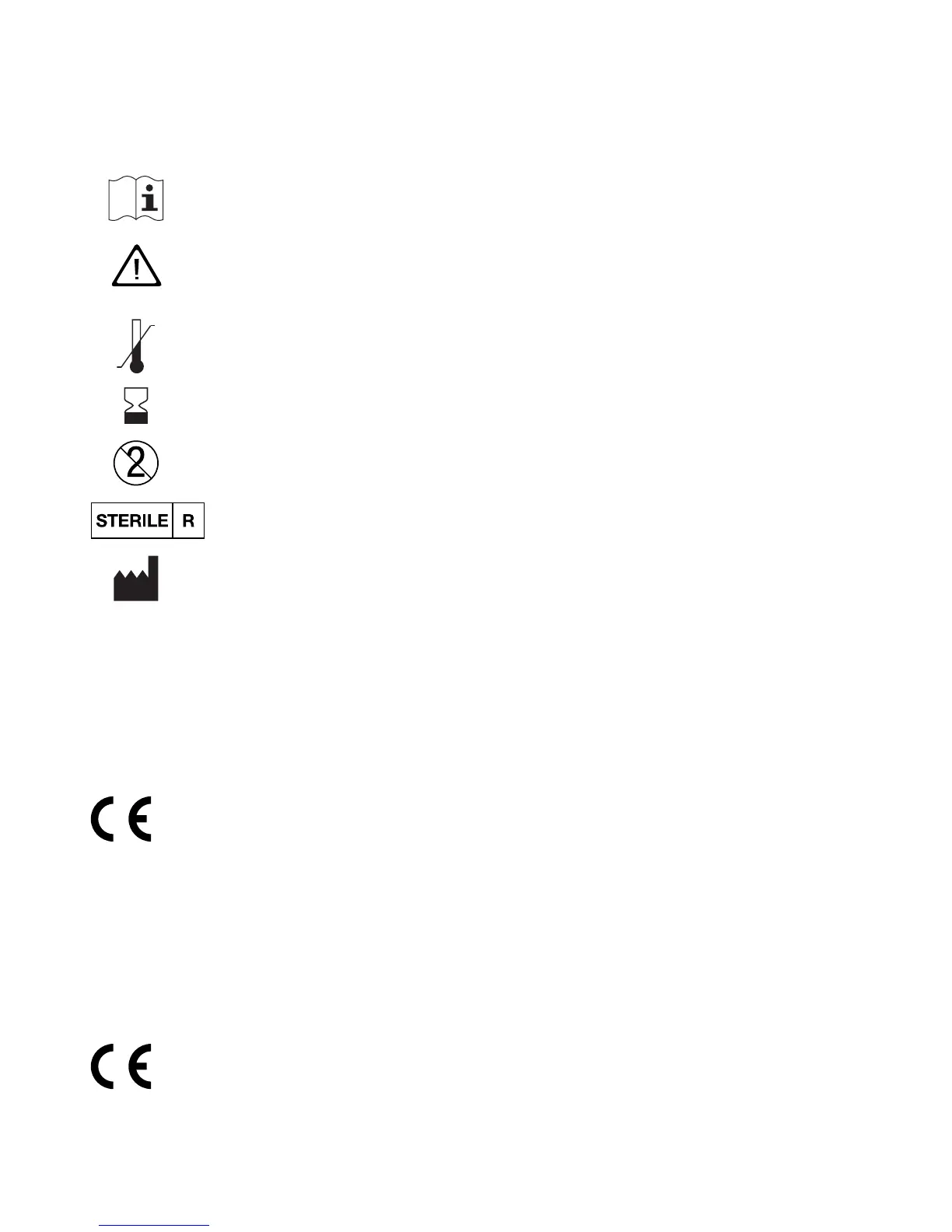
On the packaging, on the type plate of the meter and on the lancing
device you may encounter the following symbols shown below. They
have the following meanings:
Consult instructions for use
Caution, refer to safety-related notes in the instructions for
use accompanying this product.
Temperature limitation (store at)
Use by
Use only once
Sterilized using irradiation
Manufacturer
Catalogue number
Batch code
In vitro diagnostic medical device
Blood glucose meter: This product fulfils the requirements
of the European Directive 98/79/EC on in vitro diagnostic
medical devices.
Lancing device and lancets: These products fulfil the
requirements of the European Directive 93/42/EEC on
medical devices.
AST cap: This product fulfils the requirements of the
European Directive 93/42/EEC on medical devices.
0088
Farbe: P 249 – Prüfmittelnummer: 203
R
L
I
man_06919570001_01_EnCan.indd 2 18.01.2013 18:37:56

 Loading...
Loading...