GE HEALTHCARE PROPRIETARY TO GE
D
IRECTION 5394227, 12 LOGIQ S8/LOGIQ E8 SERVICE MANUAL
Section 4-4 - Functional Checks 4 - 45
4-4-18-3 Appearance Inspection
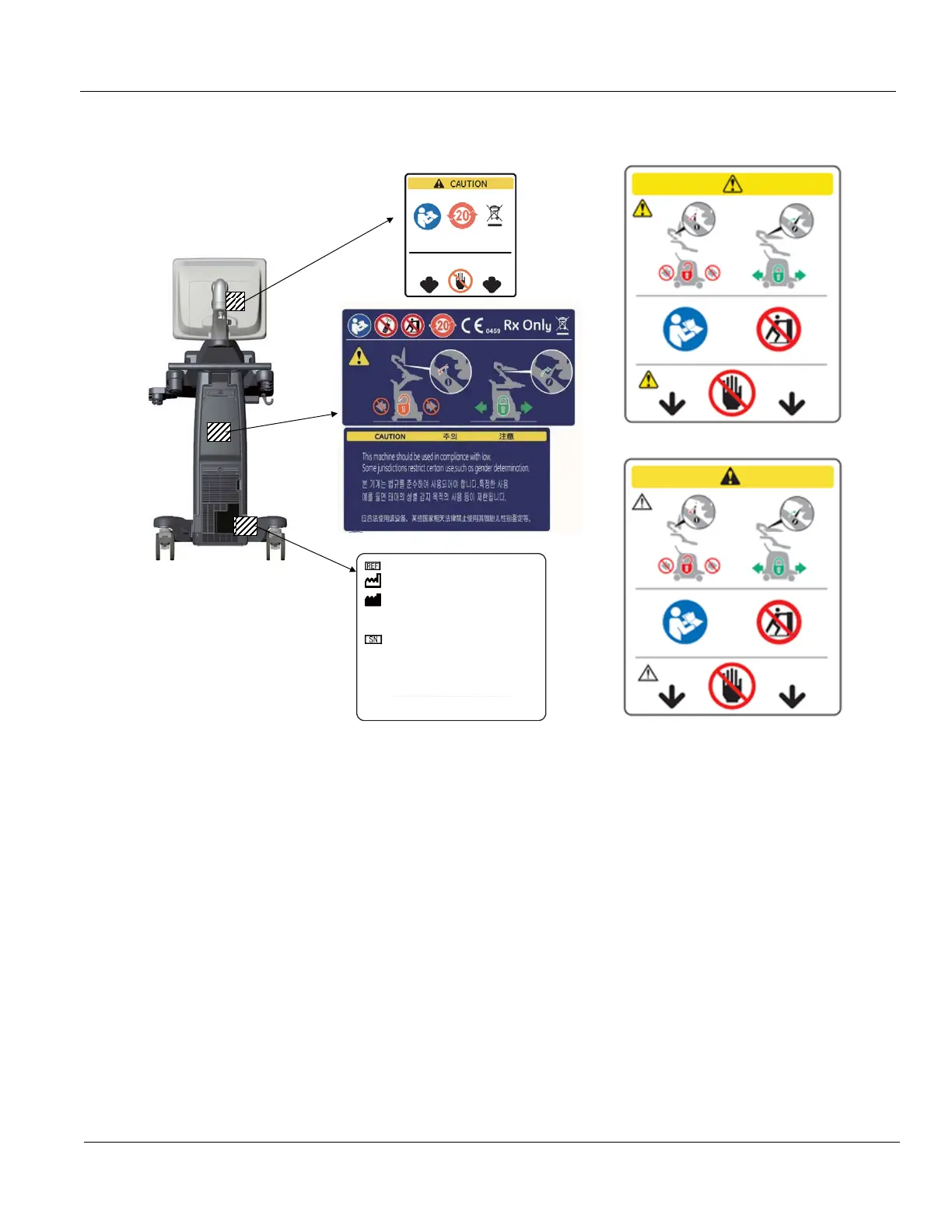
Figure 4-34 Product Labels
1.) Check presence of Caution Label on the rear panel of the monitor (2-3).
2.) Check presence of Multi Caution Label (2-2).
3.) Check presence of Rating Plate (2-1).
NOTE: Rating Plate design slightly differ depending on Console destination. At minimum simply check Console
number, manufacturing date and serial Number.
NOTE: Some countries may not have “gender determination” label.
<< Console No.>>
ٻ
<<
Manufacturing date >>
GE ULTRASOUND KOREA Ltd.
65-1,SANGDAEWON-DONG,JUNGWON-KU,
SEONGNAM-SI,GYEONGGI-DO,KOREA
ٻ
<< Serial No.>>
100-120V/220-240V~
900VA
50/60Hz
LOGIQ S8
BAR CODE
(2-3)
(2-2)
(2-1)
For 19” monitor
For 23” wide monitor
For 22” wide monitor (OLED)

 Loading...
Loading...