Do you have a question about the Mindray BeneHeart D20 and is the answer not in the manual?
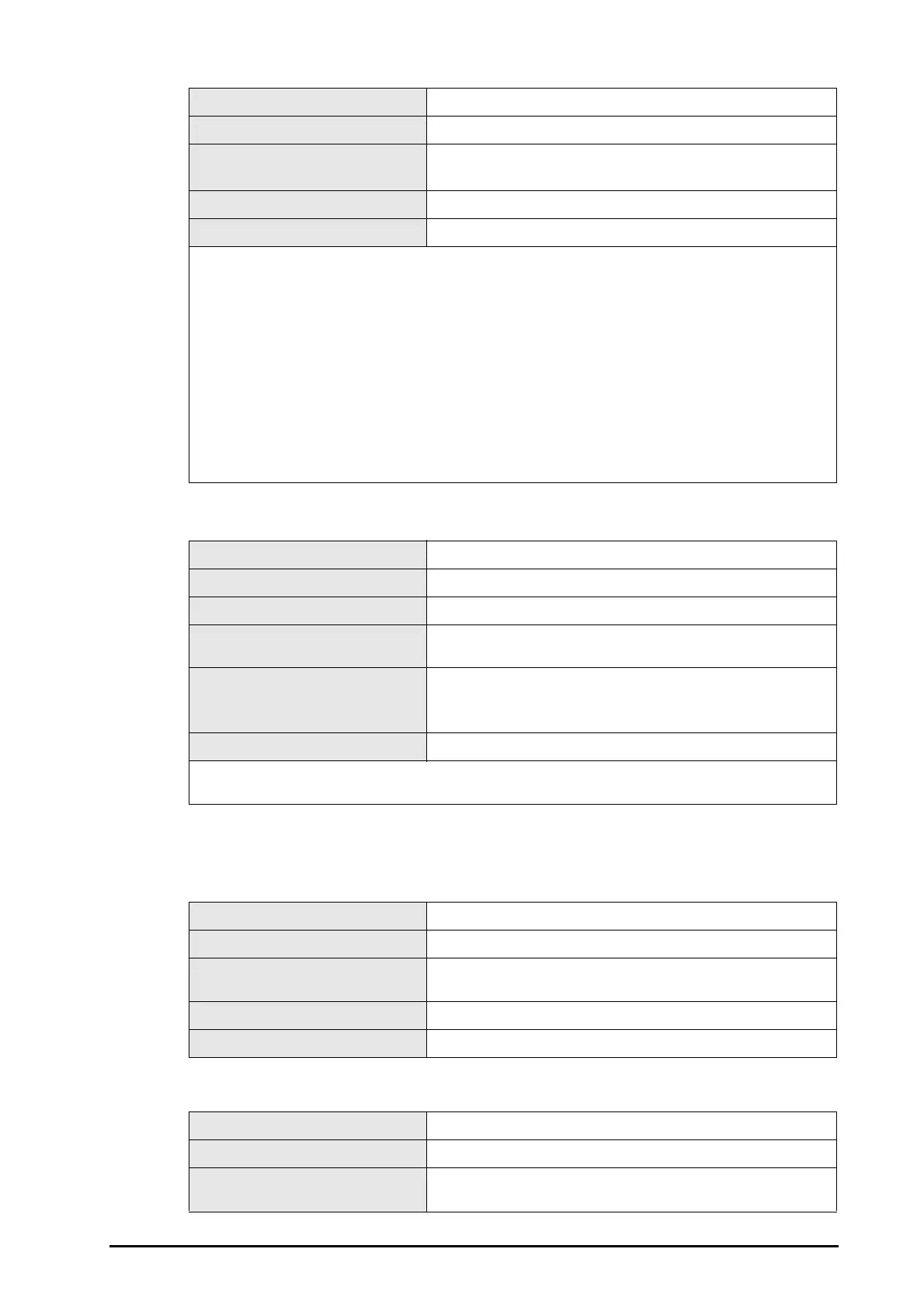
| Model | BeneHeart D20 |
|---|---|
| Connectivity | USB |
| Operating Temperature | 0°C to 50°C |
| Energy Levels (Pediatric) | 50J |
| Analysis Time | <10 seconds |
| Monitoring Parameters | ECG |
| NIBP Measurement | Not available |
| SpO2 Measurement | Yes |
| Temperature Measurement | Not available |
| Voice Prompts | Yes |
| CPR Guidance | Yes |
| Data Recording | Yes |
| Data Transfer | USB |
| Battery Type | Lithium-ion battery |
| Storage Temperature | -20°C to 60°C |
Details general safety guidelines, including DANGER, WARNING, CAUTION, and NOTE classifications.
Highlights immediate hazards that can cause death or serious injury if not avoided.
Defines the intended purpose and applications of the medical equipment.
Specifies the equipment's intended functions: defibrillation, cardioversion, pacing, and monitoring.
Lists the specific medical conditions and patient types for which each function is indicated.
Identifies the qualified medical personnel responsible for operating the equipment.
Specifies conditions where AED and Manual Defibrillation modes should not be used.
Provides critical safety warnings and cautions for setting up the equipment before use.
Details procedures and safety precautions for connecting the equipment to a power source.
Covers safety precautions, dangers, and warnings specific to Automated External Defibrillator (AED) use.
Outlines the step-by-step procedure for using the AED function, including pad placement.
Details safety information, dangers, warnings, and cautions for manual defibrillation operations.
Describes the procedures for external defibrillation using electrode pads and paddles.
Details the steps for performing synchronized cardioversion and shock delivery.
Provides safety guidelines and warnings for using the CPR assistance features.
Covers crucial safety information, warnings, and cautions for noninvasive pacing.
Provides essential safety warnings and precautions related to alarm configuration and management.
Covers safety warnings and precautions related to ECG monitoring.
Provides information and safety precautions for monitoring cardiac arrhythmias.
Covers safety warnings and precautions for respiration monitoring.
Provides essential safety warnings and precautions for SpO2 monitoring.
Covers critical safety warnings and precautions for NIBP measurements.
Provides safety warnings and precautions for CO2 monitoring.