Customer assistance
LOGIQ V3/V5/V5 Expert – Basic Service Manual 1-31
5726264-100 English Rev.8
1-11-4 Authorized Representative
1-11-5 Factory Site
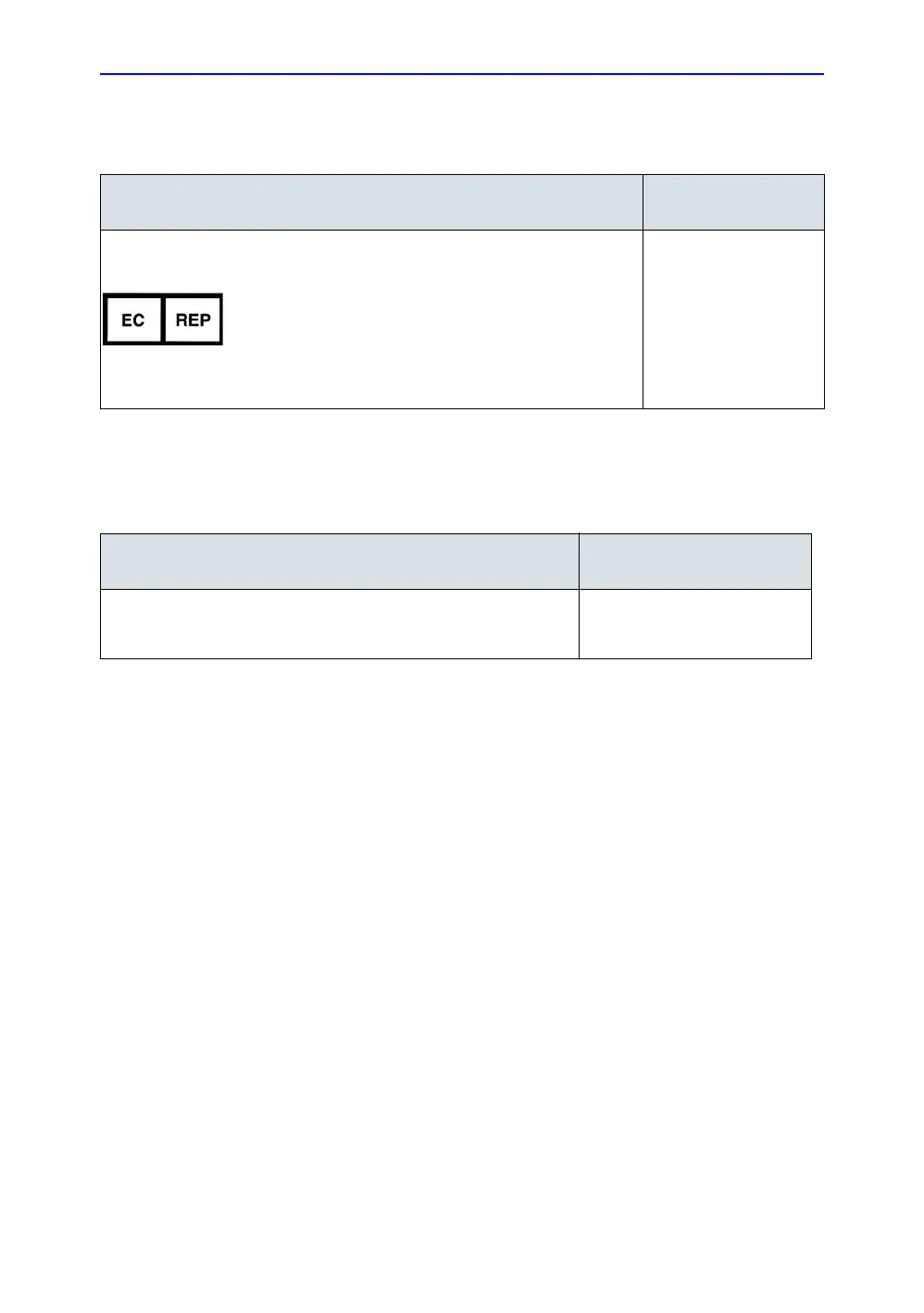
Table 1-8: Authorized Representative
AUTHORIZED REPRESENTATIVE
TELEPHONE / FAX
NUMBER
The location of the CE marking is shown in the Safety chapter of the Basic User
Manual.
Authorized EU Representative/European registered place of business:
GE Medical Systems Information Technologies GmbH (GEMS IT GmbH)
Munzinger Strasse 5, D-79111 Freiburg, GERMANY
+49 761 45 43 -0 /
+49 761 45 43 -233
Table 1-9: Factory Site
Factory Site
TELEPHONE / FAX
NUMBER
GE Medical Systems (China) Co., Ltd.
No.19, Changjiang Road, Wuxi National Hi-Tech Development Zone,
Jiangsu, P.R. China 214028
TEL: +86 510-85225888
FAX: +86 510-85226688
 Loading...
Loading...