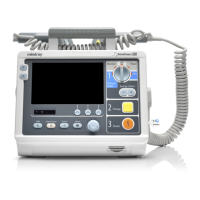
3 - 1
3 Equipment Preparation
3.1 Equipment Preparation Safety Information
• Use only installation accessories specified by Mindray.
• Connect only approved devices to this equipment. Devices connected to the equipment must meet
the requirements of the applicable IEC standards (e.g. IEC 60950 safety standards for information
technology equipment and IEC 60601-1 safety standards for medical electrical equipment). The
system configuration must meet the requirements of the IEC 60601-1 medical electrical systems
standard. Any personnel who connect devices to the equipment’s signal input/output port are
responsible for providing evidence that the safety certification of the devices has been performed in
accordance to the IEC 60601-1. If you have any questions, please contact Mindray.
• The equipment and accessories connected to the equipment are suitable for use within the patient
environment. For other devices and accessories connected to the equipment, consult corresponding
manufacturers for the suitability within the patient environment.
• If it is not evident from the equipment specifications whether a particular combination with other
devices is hazardous, for example, due to summation of leakage currents, please consult the
manufacturer or an expert in the field. A determination must be made that the proposed
combination will not negatively affect the devices themselves or the patient's safety.
• The equipment should be installed by authorized Mindray personnel.
• When disposing of the packaging material, be sure to observe the applicable waste control
regulations and keep it out of children’s reach.
• Before use, verify whether the packages are intact, especially the packages of single use accessories.
In case of any damage, do not apply it to patients.
• Make sure that the equipment operating environment meets the specific requirements. Otherwise
unexpected consequences, e.g. damage to the equipment, could result.
• Put the equipment in a location where you can easily view and operate the equipment.
• Save the packing case and packaging material as they can be used if the equipment must be
reshipped.
3.2 Connecting the Power Supply
• Always use the accompanying power cord delivered with the equipment.
• Before connecting the equipment to the power supply, check that the voltage and frequency ratings
are the same as those indicated beside the power input of the equipment.
• Use the battery if the integrity of the protective earth conductor or the protective earthing system in
the installation is in doubt.
• Use only the specified DC/AC inverter.
 Loading...
Loading...