8 . Lambda 365 Users Guide
Basic Theory
Electromagnetic radiation can pass through most organic and inorganic compounds.
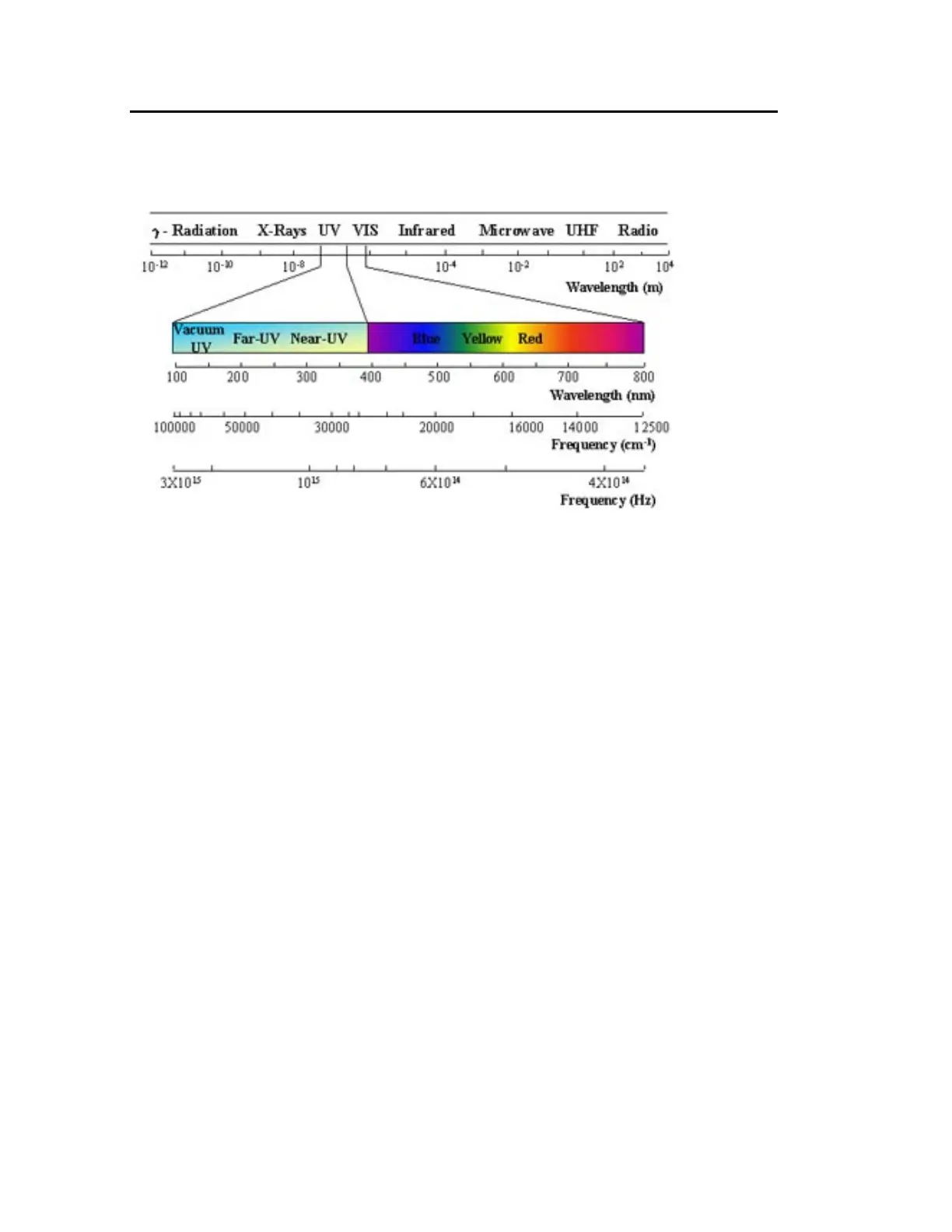
Generally, the UV range is from 190 to 380 nm and visible range is from 380-800 nm.
Figure 1. Electromagnetic Radiation
When continuous radiation passes through a sample, some is absorbed by the sample. An
absorption spectrum can be obtained by monitoring the radiation that penetrates the sample
and reaches a detector. For every substance, the absorption rate varies depending on the
wavelength of the radiation. Data from absorption spectra can be used for qualitative and
quantitative analysis.
A molecule remains in its ground state when it is stable, but it can transition to an excited
state when light energy is absorbed. This is termed absorption. (Figure. 2) When the excited
molecule returns to the ground state, it emits heat, radiation, fluorescence or
phosphorescence. This is termed emission. A molecule whose functional group has a double
bond between carbons or between carbon and another atom undergoes transitions in the
UV-Visible range. Functional groups that absorb light energy are called Chromophores.

 Loading...
Loading...