Introduction
Information relating to intended use
1
LUCEA 50-100
IFU 01741 EN 11
21 / 60
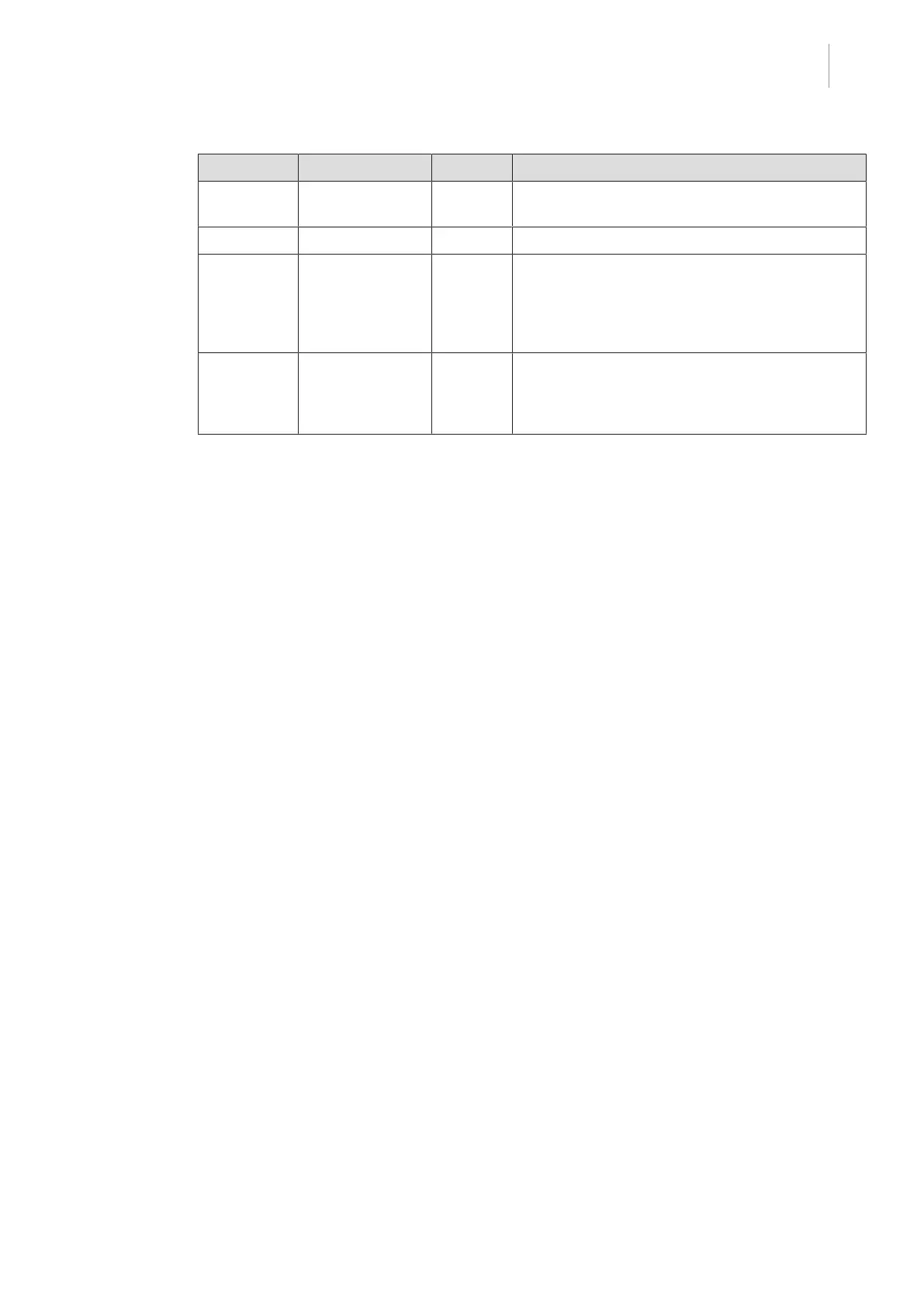
Country Reference Year Title
Taiwan TPAA
2018-01-31
2018 Taiwanese Pharmaceutical Affairs Act
UK Act 2021 Medical Devices Regulations 2002 No.618
USA 21CFR Part 7 2022 Title 21--Food And Drugs
Chapter I--Food and Drug Administration Depart-
ment of Health and Human Services
Subchapter A -- General
PART 7 - Enforcement policy
USA 21CFR
Subchapter H
2022 Title 21--Food And Drugs
Chapter I--Food and Drug Administration Depart-
ment of Health and Human Services
Subchapter H -- Medical Devices
Tab.7: Compliance with market standards
Other information (for China only)
产品名称:手术无影灯
规格型号:见标签
医疗器械注册证编号:国械注进20192010303
产品技术要求编号:国械注进20192010303
产品组成:由灯头(含发光二极管灯泡、调光器、灯罩)、电源箱、支架、手术灯头吊臂、摄像头
(选配,后缀带V的型号适用)及其遥控器(选配,后缀带V的型号适用)组成。
适用范围:该产品为吊顶式安装,供医疗单位作医用手术照明用。
禁忌症:无。
生产日期:见标签
使用期限:10年
注册人/生产企业名称:Maquet SAS 迈柯唯股份有限公司
注册人/生产企业住所:Parc de Limère Avenue de la Pomme de Pin CS 10008 Ardon 45074 Or-
léans Cedex 2-FRANCE
生产地址:Parc de Limère Avenue de la Pomme de Pin CS 10008 Ardon 45074 Orléans Cedex
2-FRANCE
代理人名称:迈柯唯(上海)医疗设备有限公司
代理人住所:中国上海自由贸易试验区美盛路56号2层227室
代理人联系方式:800-820-0207
修订日期:见本说明书第二页
1.11 Information relating to intended use
1.11.1 Intended use
LUCEA 50 and LUCEA 100 lightheads are surgical lights designed to illuminate the body of a pa-
tient during surgical operations, diagnostics and treatment.
1.11.2 Intended users
• The device may be operated only by medical staff who have read this manual.
• The device must be cleaned by qualified personnel.
1.11.3 Indications
The LUCEA 50-100 range is intended to be used for any type of surgery, treatment or examina-
tion requiring a specific type of lighting.

 Loading...
Loading...