Do you have a question about the Dräger AGS and is the answer not in the manual?
| Manufacturer | Dräger |
|---|---|
| Application | Anaesthetic Gas Scavenging |
| Vacuum Source | Central Vacuum System or Vacuum Pump |
| Overpressure Relief | Yes, integrated |
| Type | Active |
| Compatibility | Compatible with most anesthesia machines |
| Function | To remove waste anaesthetic gases from the operating room |
| Safety Features | Overpressure relief |
Lists trademarks owned by third-party manufacturers.
Defines key safety terms like WARNING, CAUTION, and NOTE.
Defines user groups and their responsibilities for product operation.
Specifies the system's purpose and scope of application.
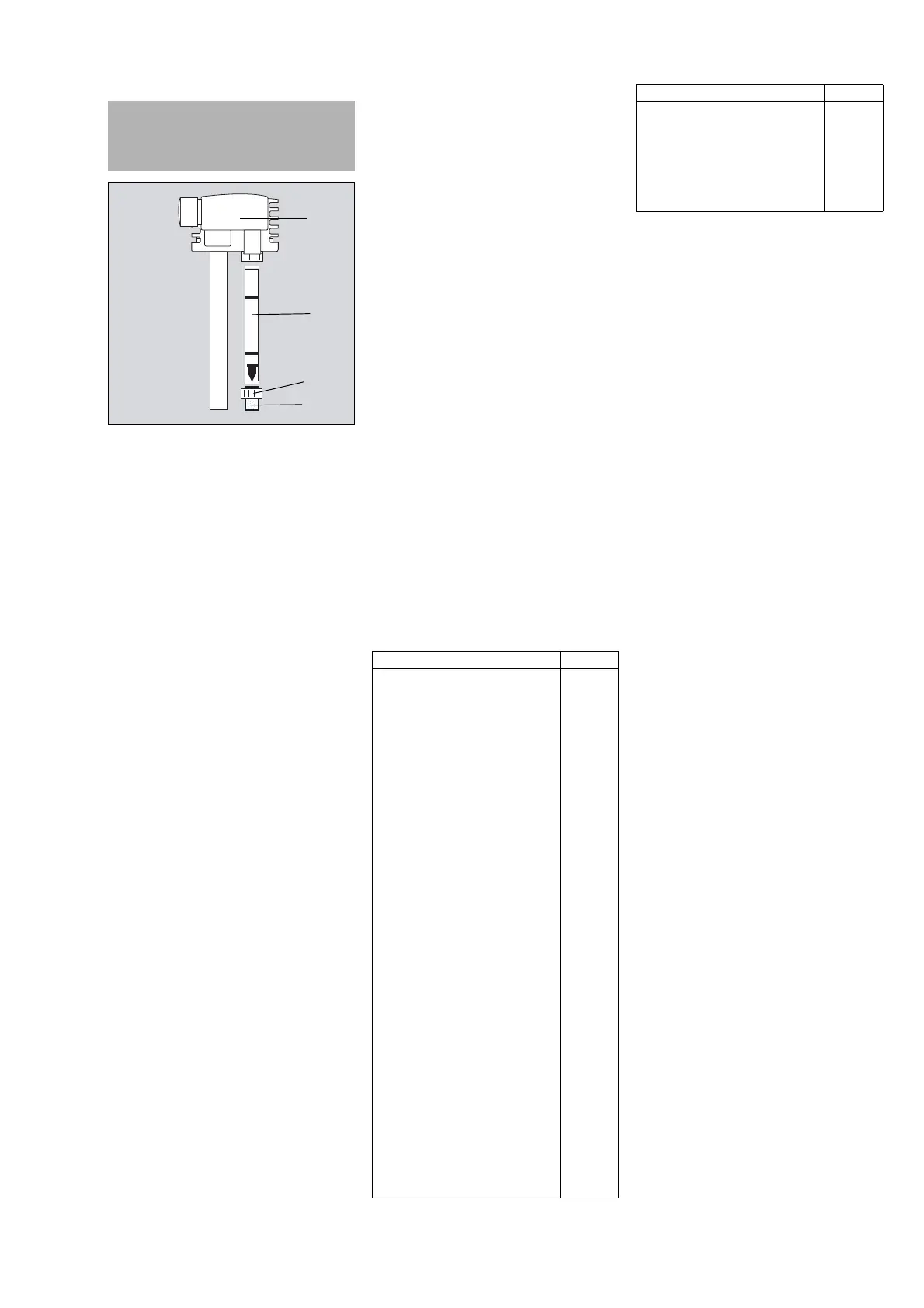
Provides a labeled diagram of the AGS system components.
Covers risks of misuse, malfunction, improper service, and incompatible accessories.
Addresses risks related to explosion hazards, fire, and reporting adverse events.
Explains AGS as part of AGSS, lists symbols and abbreviations.
Details mounting the AGS on rails or basic devices.
Instructions for connecting scavenging hoses, transfer hoses, and sample gas lines.
Specific instructions for the AGS control valve in North America.
Emphasizes performing self-tests and checking the flow tube before use.
Instructs users to check suction flow via the float position during operation.
Details the procedure for disconnecting the scavenging hose.
Highlights risks associated with improper reprocessing and product damage.
Outlines steps before reprocessing, including accessory removal.
Step-by-step guide for cleaning device surfaces with disinfectant.
Procedures for cleaning the AGS using a washer-disinfector.
Guidelines for storing and transporting the product after reprocessing.
Lists compatible disinfectants and their verification spectra.
Steps to verify the AGS is ready for operation.
Defines service terms and outlines inspection, maintenance, and repair responsibilities.
Detailed instructions for replacing the particle filter and flow tube.
Guidance on proper disposal of the AGS and its components.
Specifications for ambient conditions, performance metrics, and operating parameters.
Details dimensions, weight, medical device classification, and UMDNS code.
Lists part numbers for AGS components and related accessories.
Provides contact and address information for Drägerwerk AG & Co. KGaA and its distributors.
Directs users to the website for finding local representatives.