GE HEALTHCARE
DIRECTION 5394227, 12 LOGIQ S8/LOGIQ E8 SERVICE MANUAL
1 - 10 Section 1-3 - Important conventions
1-3-3 Product Icons
The following table describes the purpose and location of safety labels and other important information
provided on the equipment.
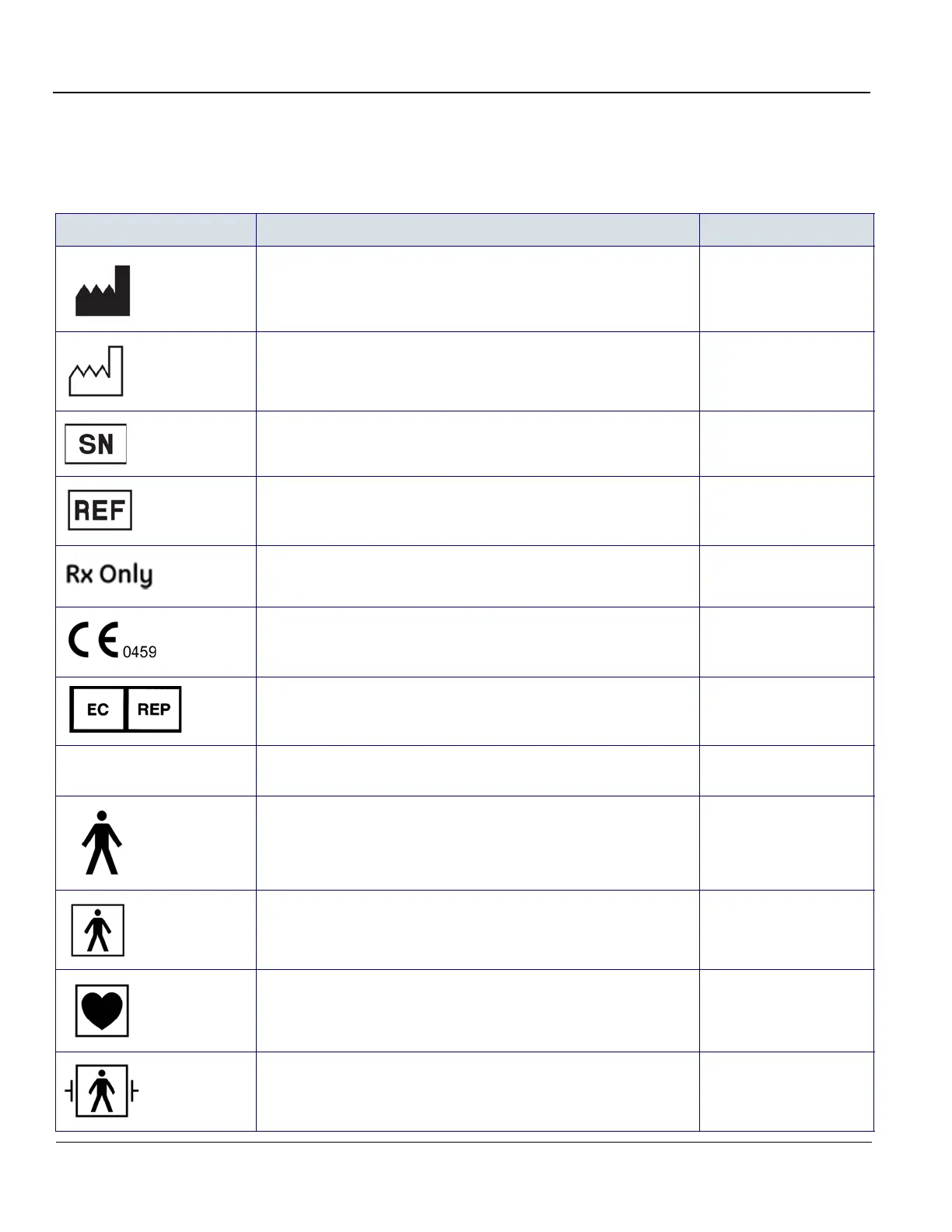
Table 1-7 Label Icons
Label/Icon Purpose/Meaning Location
Identification and Rating Plate
Manufacturer’s name and address
Rating Plate
Identification and Rating Plate
Date of manufacture
Rating Plate
Serial Number Rating Plate
Catalog Number Rating Plate
United States only
Prescription Requirement label
Rear of the system
CE Mark
The CE Mark of Conformity indicates this equipment conforms with
the Council Directive 93/42/EEC.
Rear of the system
Authorized European Representative address Rear of the system
IP Code (IPX8)
Indicates the degree of protection provided by the enclosure per
IEC60 529. Can be used in operating room environment.
Footswitch
Type B Applied Part symbol is in accordance with IEC 60878-02-03.
Probe marked Type B
Type BF Applied Part (man in the box) symbol is in accordance with
IEC 60878-02-03.
Probe marked Type BF
Defibrillation-proof applied part type BF. ECG connector
Type CF Applied Part (heart in the box) symbol is in accordance with
IEC 60878-02-03.
eTrax Needle

 Loading...
Loading...