GE HEALTHCARE
DIRECTION 5394227, 12 LOGIQ S8/LOGIQ E8 SERVICE MANUAL
Section 1-4 - Safety Considerations 1 - 21
.
CAUTION
Please observe that some printers may not be medical devices! If the Bluetooth Printer and/or
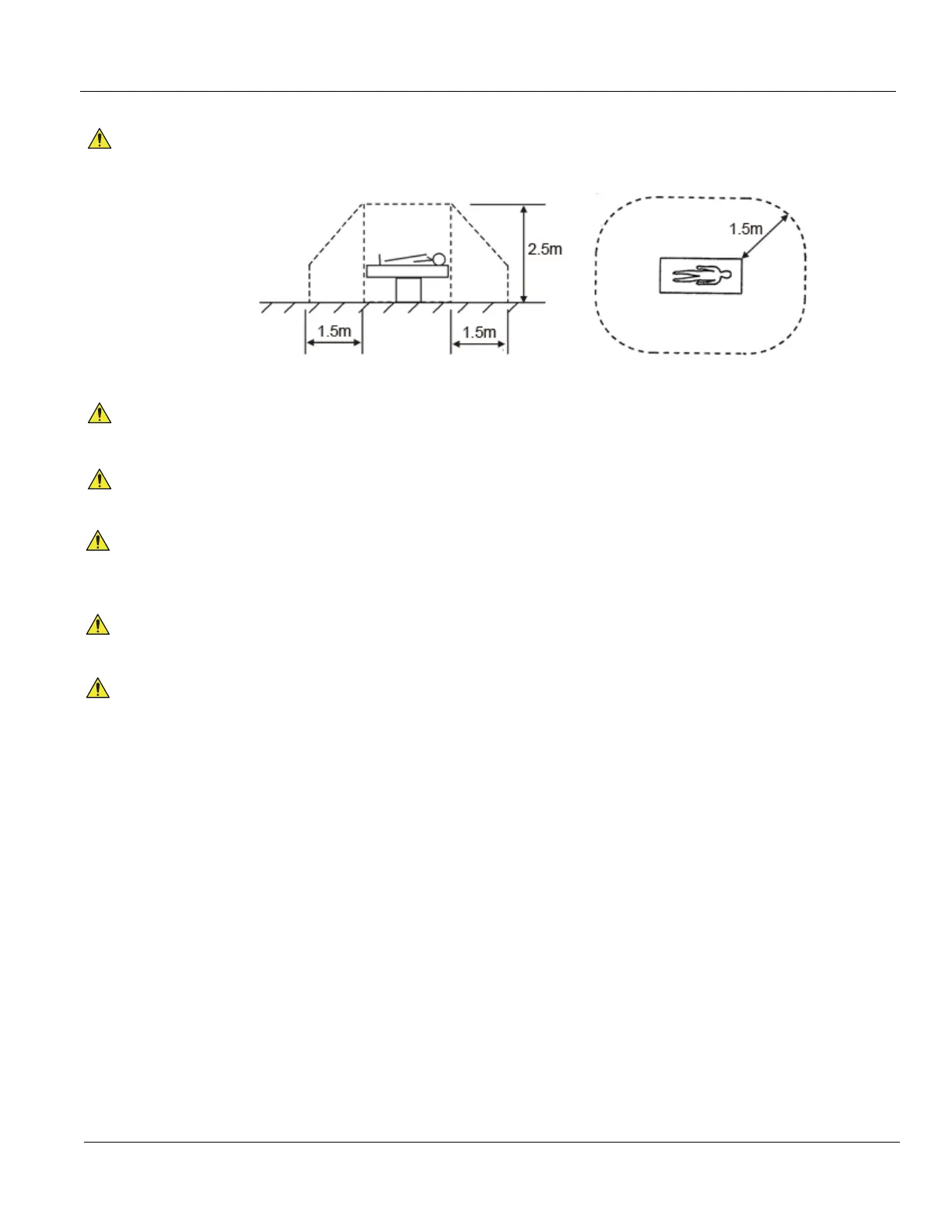
Line Printers are no medical devices, they have to be located outside of the patient environment
(according to IEC 60601-1 / UL 60601-1).
CAUTION
Auxiliary equipment must only be connected to the main console with the special main outlet
provided for the electrical safety of the system.
CAUTION
Auxiliary equipment with direct main connection requires galvanic separation of the signal and/
or control leads.
NOTICE
The system integrator (any person connecting the medical device to other devices) is responsible
that the connections are safe.
If in doubt, consult the technical service department or your local representative.
NOTICE
All peripherals mounted on the LOGIQ S8 system chassis must be firmly secured in position.
NOTICE
Each signal output (DVI, D-SUB (VGA), S-VIDEO, COMPOSITE (BNC), COMPOSITE (RCA)) of UVC
(Universal Video Converter) is insulated.
 Loading...
Loading...