AB2200en_SARev.: 04 / 11.2023
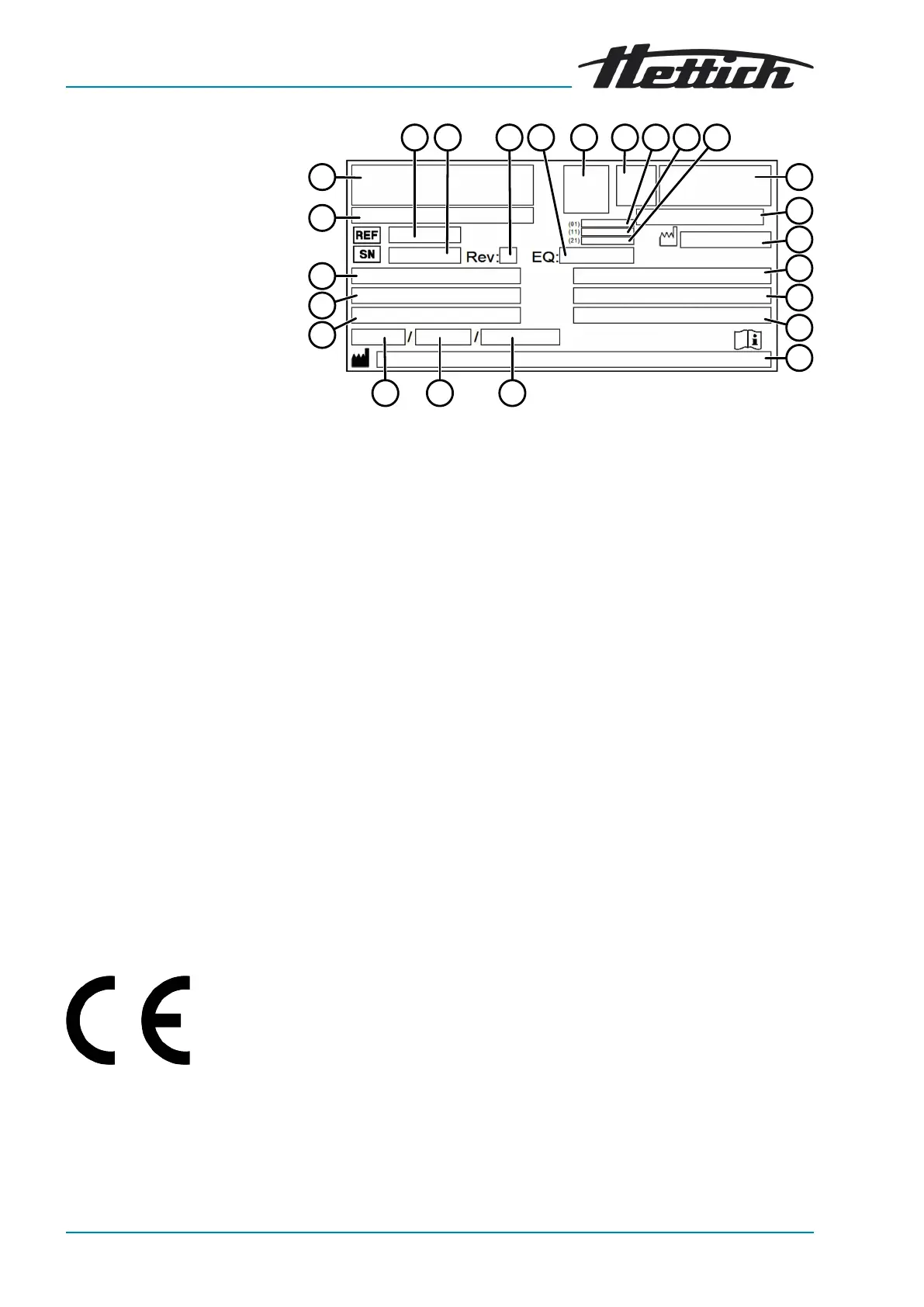
Rating plate
Device conformity
Single Registration Number
23
10
11
17
12
13
15
14
16
1819
24
20
22
21
2 43 5 6 7 8 91
Fig. 1: Rating plate
1 Item number
2 Serial number
3 Revision
4 Equipment number
5 Data matrix code
6 any labelling indicating whether medical device or in vitro diagnostic
medical device
7 Global Trade Item Number (GTIN)
8 Date of manufacture
9 Serial number
10 any EAC mark, CE mark
11 Country of manufacture
12 Date of manufacture
13 Mains frequency
14 Maximum kinetic energy
15 Maximum permissible density
16 Manufacturer's address
17 any Coolant circuit pressure
18 any Coolant capacity
19 any Coolant type
20 Revs per minute
21 Performance values
22 Mains voltage
23 any Device designation
24 Manufacturer's logo
3.2
European registration
Device conformity according to EU directives.
SRN: DE-MF-000010680

 Loading...
Loading...










