Appendix
- 40 -
MXD70 Dissolved Oxygen
Setup and Operating Guide
Appendi
Partial Pressure of Oxygen (pO2)
The concentration of a gas dissolved in a solution at equilibrium is proportional to the partial pressure
of the gas in contact with the solution (Henry’s Law). The partial pressure of the gaseous component
of the air in contact with the solution remains proportional to the total pressure of the air sample.
The partial pressure of Oxygen in air at atmospheric pressure of 1 Bar (1000mBar) is 210mBar (air is
21% Oxygen), so if a solution of pure water were 100% saturated with Oxygen at atmospheric pressure
the partial pressure of Oxygen in solution would be 210mBar. e.g. 20% saturation at a pressure of 1 Bar
gives a reading of 42mBar, 50% saturation at a pressure of 3 Bar gives a reading of 315mBar.
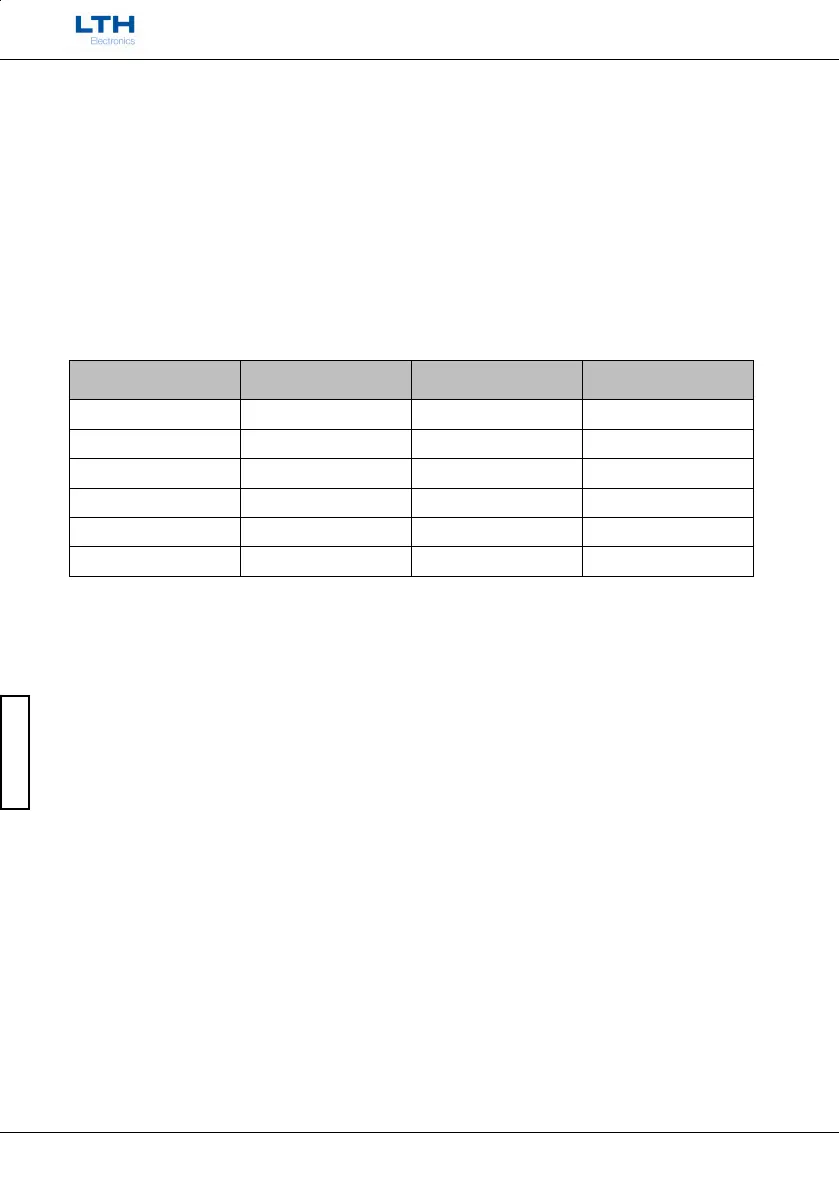
Sensor Parameters
The following table gives the necessary configuration data for a number of Dissolved Oxygen Sensors.
Sensor Type
Temperature Sensor
Type
Membrane Correction
Factor
Bias Voltage
LTH OE15 1K Thermistor 3965 N/A
BJ ProcessProbe™ 22k Thermistor 2220 +0.675
Hamilton Oxysens™ 22k Thermistor 2700 +0.670

 Loading...
Loading...