Section 4: Units & Conversions
Fundamental Topics in Science © 2001 Texas Instruments Teacher Notes 4-4
Introduction
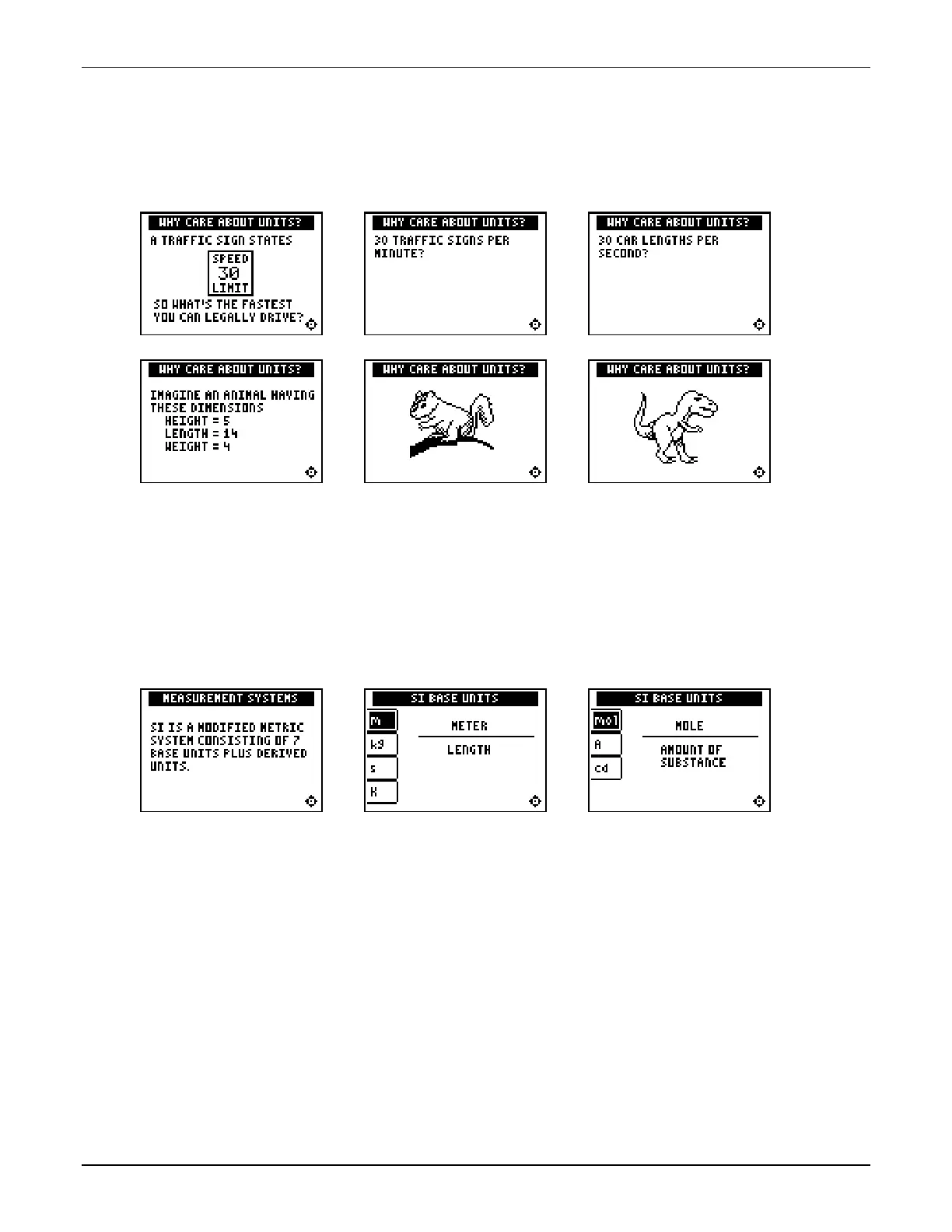
This section begins by exploring the idea that it is meaningless to describe a physical quantity
without including its associated unit of measurement. Two examples are given.
Concepts
This section addresses the idea of standard measuring systems and introduces the SI
measurement system. Students probably are comfortable with measurements of length, mass,
time, and temperature, but less familiar with amount of substance, electric current, and
luminous intensity. You may want to discuss the types of measurements where those units
would be used. Even though these units may not be studied in detail in your course, we include
them because these seven units are the basis from which all other SI units are derived.
Students may be interested to know that SI is an acronym for “Le Systeme International
d’Unites” (the International System of Units).
Extension
The acceptance of SI as a worldwide standard offers tremendous advantages in science and
technology as well as in commerce. Ask students if they have encountered problems trying to
use two different measuring systems together. Examples they might come up with include trying
to remove an “English” nut (one specified to SAE standards) with a metric socket set (or vice
versa), trying to pour a liter bottle of juice into a quart container, or inheriting a recipe with
European measurements.
SI prefixes are used when describing very large or very small quantities. The prefixes are not
limited, however, to SI. Students are probably familiar with some of the prefixes such as giga,
mega, kilo, and nano in the context of personal computers.

 Loading...
Loading...