Requirements placed on the steam steriliser:
– Corresponds to EN 13060 or EN 285 and/or
ANSI AAMI ST79
– Suitable programme for the products listed
(e. g. with hollow bodies, fractionated vacuum
procedure in three vacuum steps)
– Sufficient product drying
– Validated process in accordance with DIN EN
ISO 17665 (valid IQ/OQ and product-specific
performance appraisal (PQ)
Perform the following steps:
❯
Sterilise the parts for sterilisation (at least 20
minutes at 121°C, at least 4 minutes at 132°C
or at least 5 minutes at 134°C).
Do not exceed 138 °C.
Marking
❯
Mark the packaged, treated medical product in
such a way as to ensure safe application.
10.8 Issue clearance for the parts
for sterilisation
The reprocessing of the medical products ends
with the documented clearance for storage and
renewed use.
❯
Document the clearance of the medical prod-
uct after reprocessing.
10.9 Storing parts for sterilisation
❯
Comply with the stated storage conditions:
– Store the parts protected against contami-
nation
– Dust-protected, e.g. in a locked cabinet
– Protected against moisture
– Protected against excessive temperature
fluctuations
– Protected against damage
Packaging for a sterile medical device can suf-
fer damage as a result of a particular incident
and the passage of time.
Potential external contamination of the sterile
barrier system should be taken into account in
terms of aseptic preparation when establishing
the storage conditions.
11 Cleaning
11.1 Cleaning the optical element
NOTICE
Damage of the optical element from
incorrect cleaning
❯
Only use the cleaning set for VistaCam
optical element. Disinfectant residues
soil the optical element.
❯
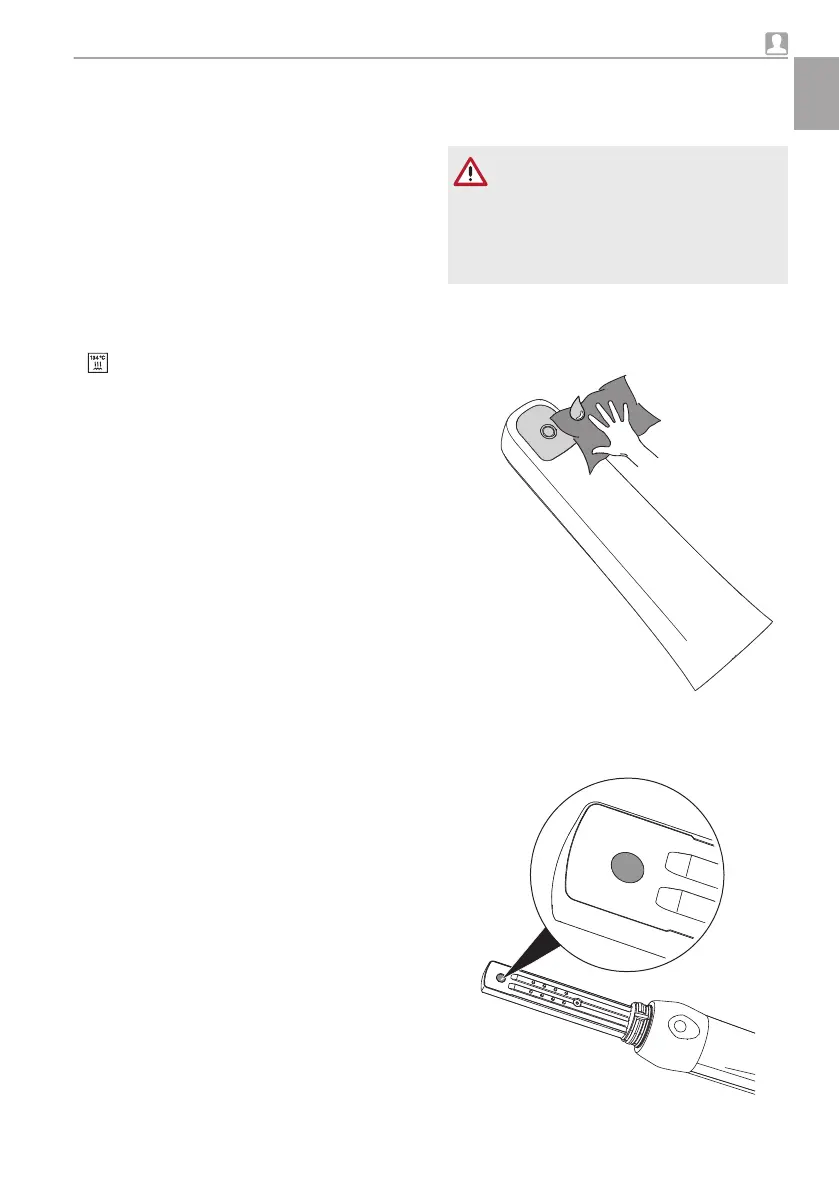
Clean the window of the optical window of the
interchangeable head from outside using the
microfibre cloth with a drop of VistaCam optical
element cleaner or alcohol.
❯
With the interchangeable head removed, clean
the surface of the image sensor using the
cleaning set for VistaCam Optik.
Usage
9000-618-176/30 1812V009 31
EN

 Loading...
Loading...











