4-4
Measurement validation
The SpO
2
accuracy has been validated in human studies against arterial blood sample
reference measured with a CO-oximeter. Pulse oximeter measurements are statistically
distributed, and only about two-thirds of the measurements can be expected to fall within
the specified accuracy compared to CO-oximeter measurements.
NOTE
The SpO
2
simulator can only be used to verify that the pulse oximeter operates
properly. It cannot be used to verify the accuracy of the pulse oximeter or the SpO
2
sensor. To verify the accuracy, clinical tests are required.
Test Method 2
Tool required:
SpO
2
simulator, Index-2 recommended
1. Connect the SpO
2
sensor to the SpO
2
simulator.
2. Selected the model and manufacturer of the SpO
2
module to be tested on the simulator,
and set the simulator as follows: SpO
2
to 96% and PR to 80 bmp.
3. Set the patient type to [Adu], [Ped], and [Neo] respectively. Observe the monitor and
make sure the displayed SpO
2
and PR value fall in the following range.
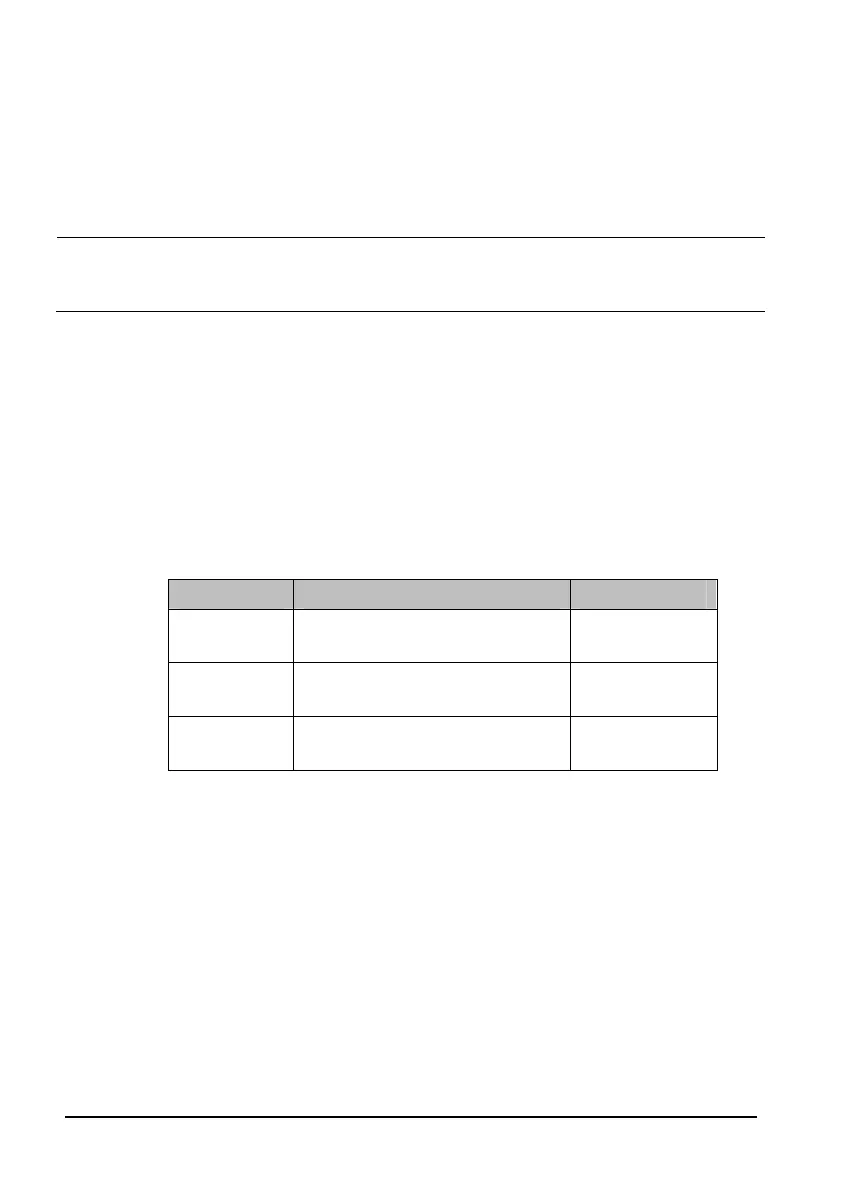
Manufacturer SpO
2
PR
Mindray
96% ± 2% (Adult, pediatric)
96% ± 3% (Neonate)
80 ± 3 bpm
Nellcor
96% ± 2% (Adult, pediatric)
96% ± 3% (Neonate)
80 ± 3 bpm
Masimo
96% ± 2% (Adult, pediatric)
96% ± 3% (Neonate)
80 ± 3 bpm
 Loading...
Loading...