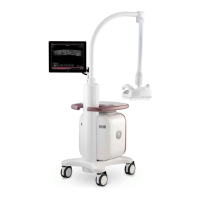
Preparing for Setup
Invenia ABUS 2.0 – System Setup and Basic Service Manual 3-39
4700-0043-00 Rev. 4
Single Use Only Single-Use Stabilization
Membrane
This symbol indicates that waste
electrical and electronic equipment
must not be disposed of as unsorted
municipal waste and must be collected
separately. Please contact an
authorized representative of the
manufacturer for information
concerning the decommissioning of
your equipment.
Rating Plate
TUV Rhineland Listing Mark
Monogram
Rating Plate
This product consists of devices that
may contain mercury, which must be
recycled or disposed of in accordance
with local, state, or country laws.
Within this device the backlight lamps
in the monitor display contain mercury.
Rating Plate
Product Label
Unique Device Identification (UDI)
Label. The UDI label consists of a
series of alpha-numeric characters
and barcode which uniquely identify
the device as a medical device
manufactured by General Electric.
Next to the Rating Plate
GE Cares label with System ID and
service phone number.
Monitor
(rear panel)
Table 3-6: Label Icons (Continued)
Label/Icon Purpose/Meaning Location
 Loading...
Loading...