GE HEALTHCAREDRAFT VOLUSON E8 / VOLUSON E6
D
IRECTION KTD102576, REVISION 7 DRAFT (AUGUST 23, 2012) SERVICE MANUAL
Chapter 1 - Introduction 1-13
1-3-5 Auxiliary Devices Safety (cont’d)
Everybody who connects additional equipment to the signal input portion or signal output portion
configures a medical system, and is therefore responsible that the system complies with the
requirements of the system standard IEC 60601-1-1.
Special care has to be taken, if the device is connected to computer network (e.g., Ethernet), because
other devices could be connected without any control. There could be a potential difference between
the protective earth and any line of the computer network including the shield.
In this case the only way to operate the system safely is to use an isolated signal link with minimum
4mm creepage distance, 2.5mm air clearance of the isolation device. For computer networks there are
media converters available which convert the electrical to optical signals. Please consider that this
converter has to comply with IEC xxx standards* and is battery operated or connected to the isolation
mains output of the Voluson E8 / Voluson E6 ultrasound system.
* IEC xxx stands for standards such as:
• IEC 60601 for medical devices
• IEC 60950 for information technology equipment etc.
NOTE: Always observe the instructions given in the manual of the peripheral/auxiliary device.
For hardware installation procedures see: Chapter 3 - Connection of Auxiliary Devices, on page 3-9.
!! NOTICE:
The system integrator (any person connecting the medical device to other devices) is responsible
that the connections are safe.
If in doubt, consult the technical service department or your local representative.
!! CAUTION:
The leakage current of the entire system including any / all auxiliary equipment must not exceed
the limit values as per EN 60601-1-1:1990 (IEC 60601-1-1) respectively other valid national or
international standards. All equipment must comply with UL, CSA and IEC requirements.
!! CAUTION:
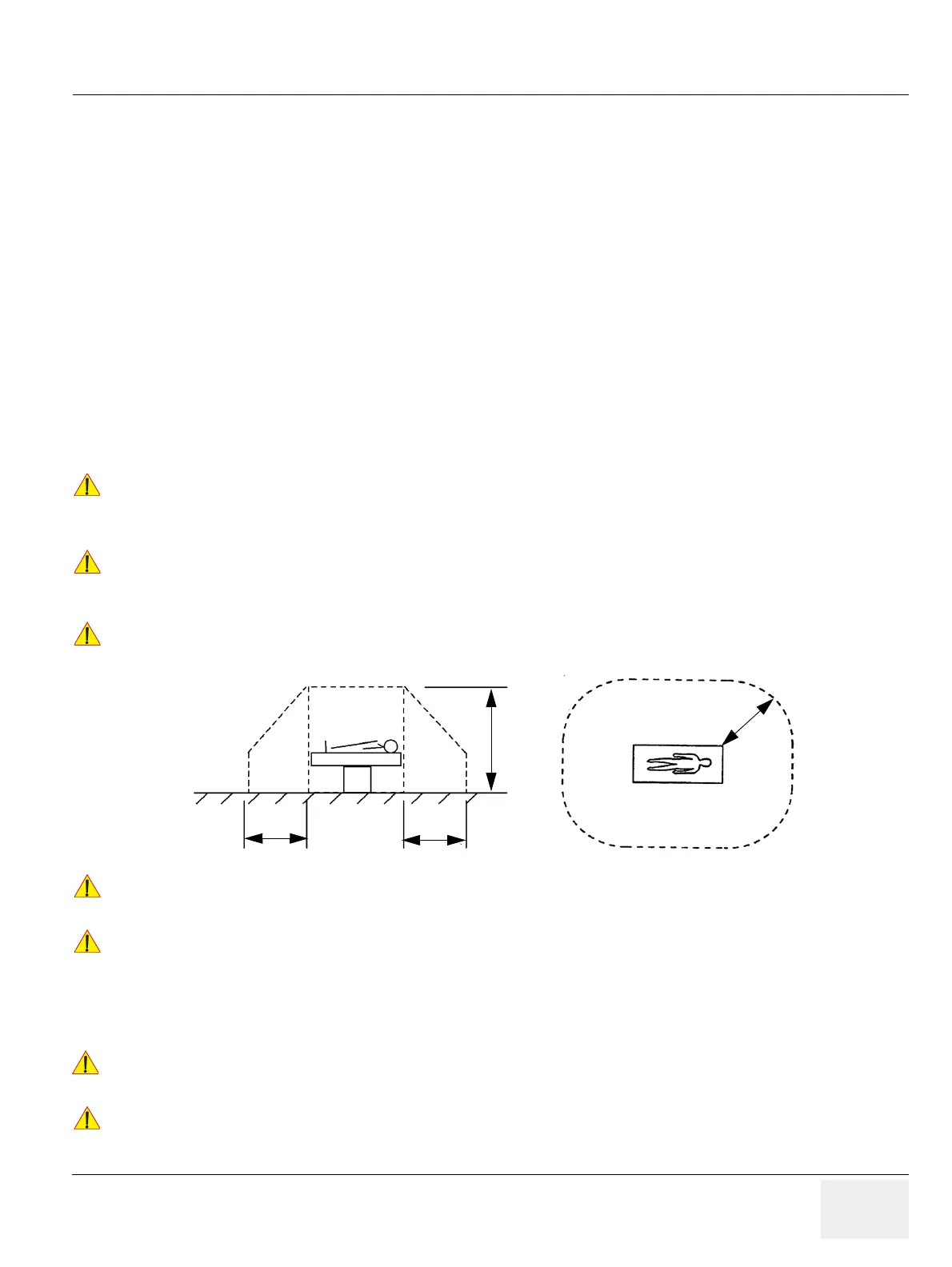
Please observe that some printers may not be medical devices! If the Bluetooth Printer and/or
Line Printers are no medical devices, they have to be located outside of the patient environment
(according to IEC 60601-1 / UL 60601-1).
!! CAUTION:
Auxiliary equipment must only be connected to the main console with the special main outlet
provided for the electrical safety of the system.
!! CAUTION:
Auxiliary equipment with direct main connection requires galvanic separation of the signal and/
or control leads.
!! WARNING:
After each installation, the leakage currents have to be measured according to
IEC 60601-1 respectively UL 60601-1.
!! NOTICE:
All peripherals mounted on the Voluson E8 / Voluson E6 system chassis must be firmly secured in
position.
 Loading...
Loading...