.1
69 / 93
7.5 Data types
Because ASTM E1394 does not support sub-components, every field that requires data type
consisting of sub-components will be defined in the Roche Diagnostics ASTM 2.0
specification explicitly. In most cases it is sufficient to consider the 1
st
sub-component of
component only.
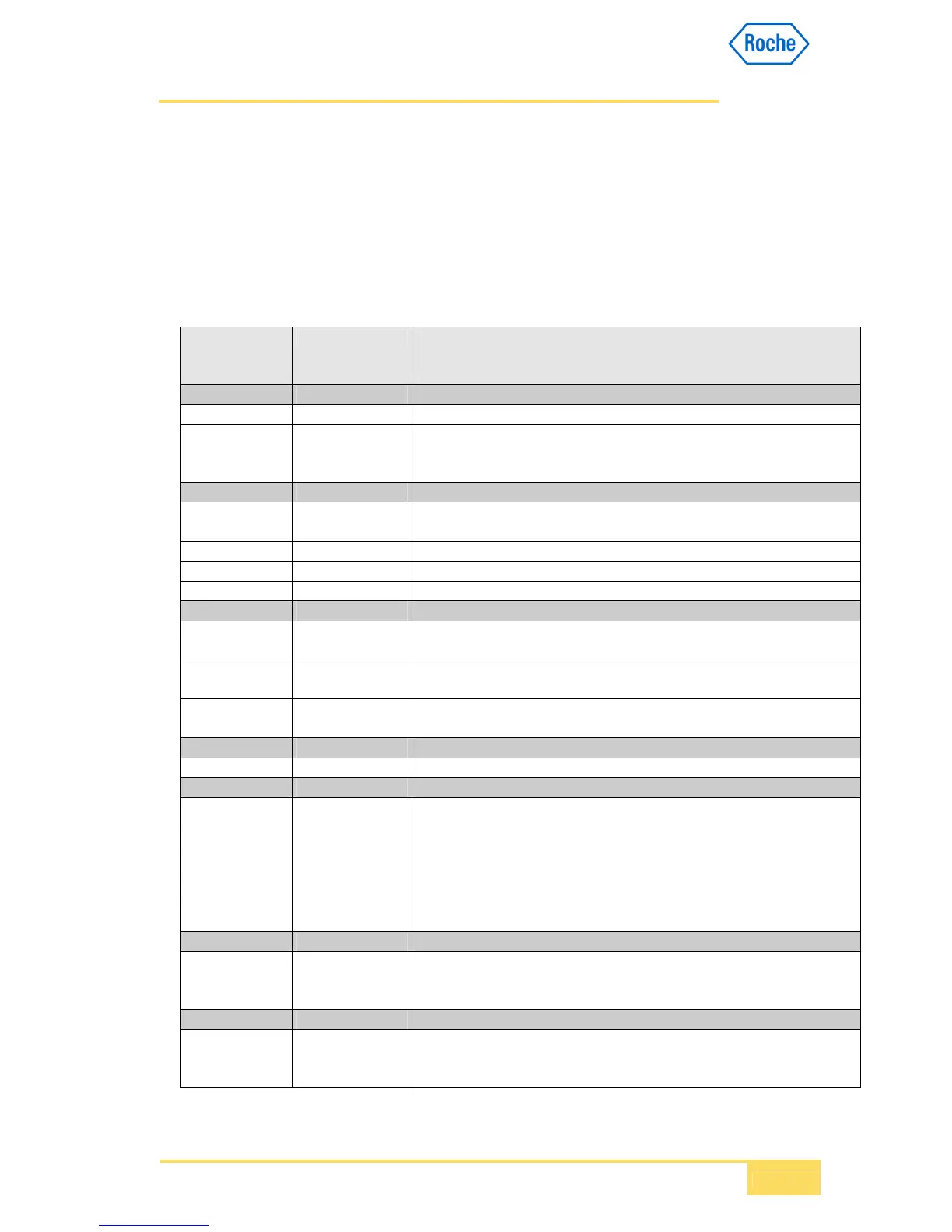
List of data types used in the Roche Diagnsotics ASTM 2.0 MSRs based on the HL7v2.4
chapter 13 is presented below. For details refer to the chapter 2 of the current version of
HL7 standard.
Data Type
Category/ Data
type
Data Type Name Notes/Format
Alphanumeric
ST String
FT Formatted text Roche ASTM does not require support for any specific formatting characters,
i.e. FT=ST. Any format characters should be send using escape delimiters as
defined in the ASTM E1394, e.g., &XA& could equal line feed.
Numerical
CQ Composite quantity
with units
<quantity (NM)> ^ <units (CE)>
NM Numeric
SN Structured numeric <comparator> ^ <num1 (NM)> ^ <separator/suffix> ^ <num2 (NM)>
NA Numeric Array <value1 (NM)> ^ <value2 (NM)> ^ <value3 (NM)> ^ ...
Identifier
ID Coded values for
HL7 tables
IS Coded value for
user-defined tables
EI Entity identifier <entity identifier (ST)> ^ <namespace ID (IS)> ^ <universal ID (ST)> ^
<universal ID type (ID)>
Date/Time
TS Time stamp YYYY[MM[DD[HHMM[SS[.S[S[S[S]]]]]]]][+/-ZZZZ] ^ <degree of precision>
Code Values
CE Coded element <identifier (ST)> ^ <text (ST)> ^ <name of coding system (IS)> ^ <alternate
identifier (ST)> ^ <alternate text (ST)> ^ <name of alternate coding system
(IS)>
Roche ASTM requires that only the 1
st
component will be implemented.
Implementation of other components is optional. Of course in case of
implementation of the 1
st
component only, the trailing component delimiters
can be omitted.
Generic
CM Composite <specimen source name or code (CE)> ^ <additives (TX)> ^ <freetext (TX)>
^ <body site (CE)> ^ <site modifier (CE)> ^ <collection method modifier
code (CE)> ^ <specimen role (CE)>
Time Series:
TQ Timing/quantity <quantity (CQ)> ^ <interval (*)> ^ <duration (*)> ^ <start date/time (TS)> ^
<end date/time (TS)> ^ <priority (ID)> ^ <condition (ST)> ^ <text (TX)> ^
<conjunction (ID)> ^ <order sequencing (*)>
 Loading...
Loading...