PA Series Biofeedback and Stimulation System User Manual EMC Information
64
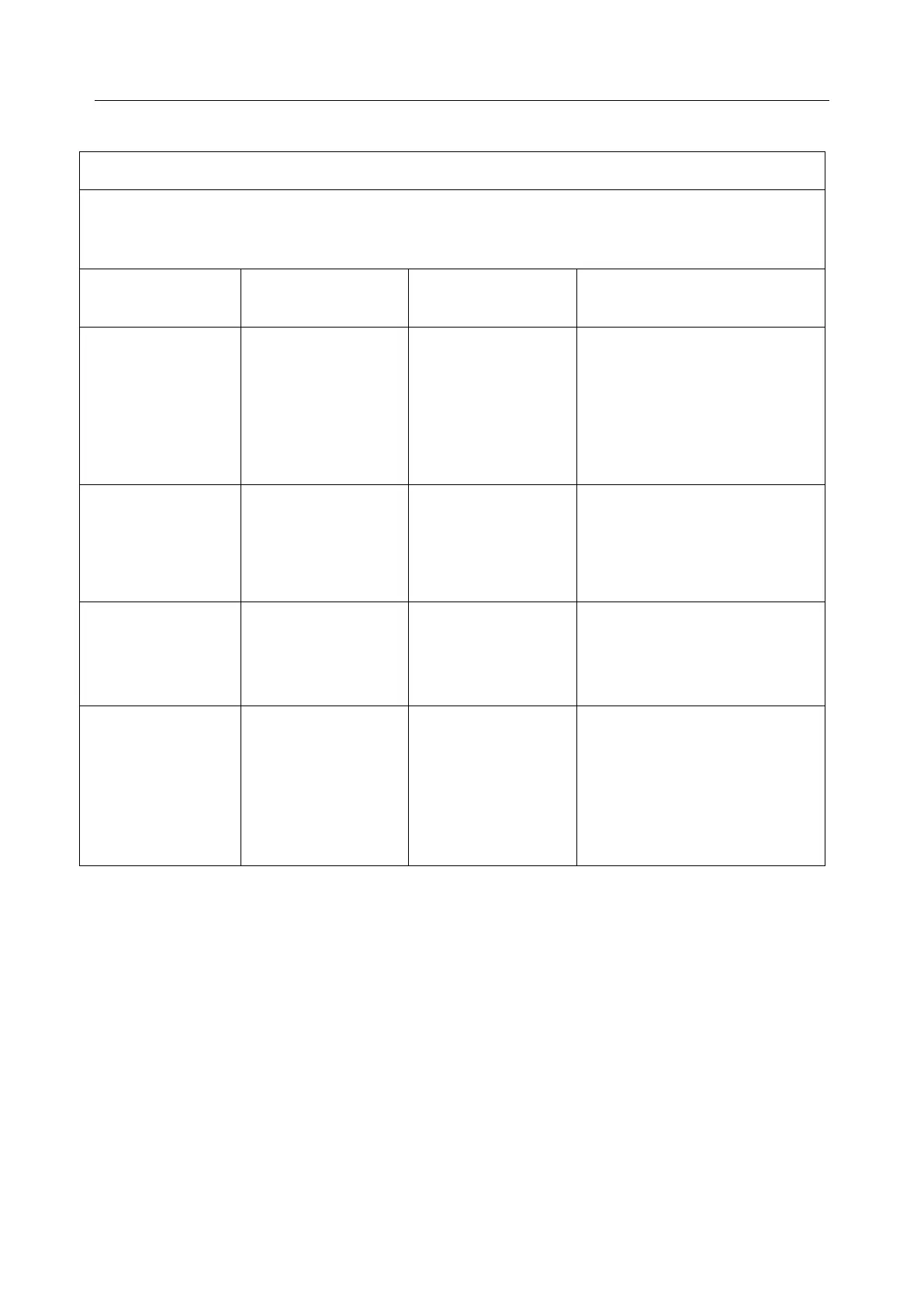
A3.2 Electromagnetic Immunity
Guidance and manufacture’s declaration – electromagnetic immunity
The PA Biofeedback and Stimulation system is intended for use in the electromagnetic environment
specified below. The customer or the user of the PA Biofeedback and Stimulation System should
assure that it is used in such an environment.
Electromagnetic environment
- guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
Floors should be wood,
concrete or ceramic tile. If floor
are covered with synthetic
material, the relative humidity
should be at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
2 kV for power
supply lines
1 kV for
input/output lines
2kV for power
supply lines
Not Applicable
Mains power quality should be
that of a typical commercial or
hospital environment.
1 kV line(s) to
line(s)
2 kV line(s) to earth
1 kV line(s) to
line(s)
2 kV line(s) to earth
Mains power quality should be
that of a typical commercial or
hospital environment.
Power frequency
(50Hz/60Hz)
magnetic field
IEC61000-4-8
Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical commercial
or hospital environment.

 Loading...
Loading...