Publication Information
Publication Information
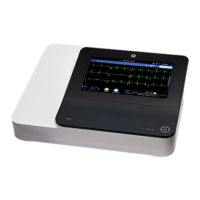
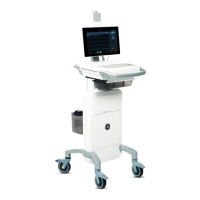
This document describes version 1.02 of the MAC VU360
™
Resting ECG Analysis System, also referred to as the “product” or "system" or
"device". It does not apply to earlier product versions. Due to continuing product innovation, specifications in this document are subject to
change without notice.
12SL, CASE, CardioSoft, InSite ExC, MAC, MACCRA, MARS, MUSE, Marquette, MobileLink, and MULTI-LINK are trademarks owned by GE
Medical Systems Information Technologies, Inc., a General Electric Company going to market as GE Healthcare. All other trademarks
contained herein are the property of their respective owners.
This product complies with the requirements concerning medical devices from the following regulatory bodies.
Date of first CE mark - 2018.
For more information about compliance, refer to the Regulatory and Safety Manual for this product.
The document part number and revision are on each page of the document. The revision identifies the document’s update level. The
revision history of this document is summarized in the following table.
Revision Date Comment
1 30 April 2020 Customer Release
2 28 February 2021 Update content for Software SP01
To access other GE Healthcare Diagnostic Cardiology documents, go to https://www.gehealthcare.com/en/support/support-documentation-
library, and click Enter Customer Documentation Portal.
To access Original Equipment Manufacturer (OEM) documents, go to the device manufacturer's website.
This document describes the MAC VU360
™
Resting ECG Analysis System, also referred to as the” product”, “system”, or “device”. This
document is intended to be used by an operator of the MAC VU360
™
Resting ECG Analysis system.
Installation should be performed by a qualified personnel.
The MAC VU360
™
Resting ECG Analysis System is intended to be used under the direct supervision of a licensed healthcare practitioner, by
trained operators in a hospital or facility providing patient care.
This document provides information required for the proper use of the system. Familiarize yourself with this information and read and
understand all instructions before attempting to use this system. Keep this document with the Regulatory and Safety manual, and with the
equipment at all times, and periodically review it.
Illustrations in this document are provided as examples only. Depending on system configuration, screens in the document may differ from
the screens on your system. Patient names and data are fictitious. Any similarity to actual persons is coincidental.
Third-party Licenses
This product includes software developed by:
• Linux Kernel organization (https://www.kernel.org)
• NXP Semiconductors (https://www.nxp.com)
• Apache Software Foundation (http://www.apache.org)
• OpenSSL.org (http://www.openssl.org)
• OpenSSH (https://www.openssh.com/)
• GNU Foundation packages (https://www.gnu.org)
• Gentoo software (https://packages.gentoo.org)
• Boost Libraries (http://www.boost.org)
• POCO Project (https://pocoproject.org)
2 MAC VU360
™
Resting ECG Analysis System 2088531-370-2
 Loading...
Loading...