Chapter 2: Safety and Regulatory
5495975-1EN Rev.9 2-42
© 2013-2017 General Electric Company. All rights reserved.
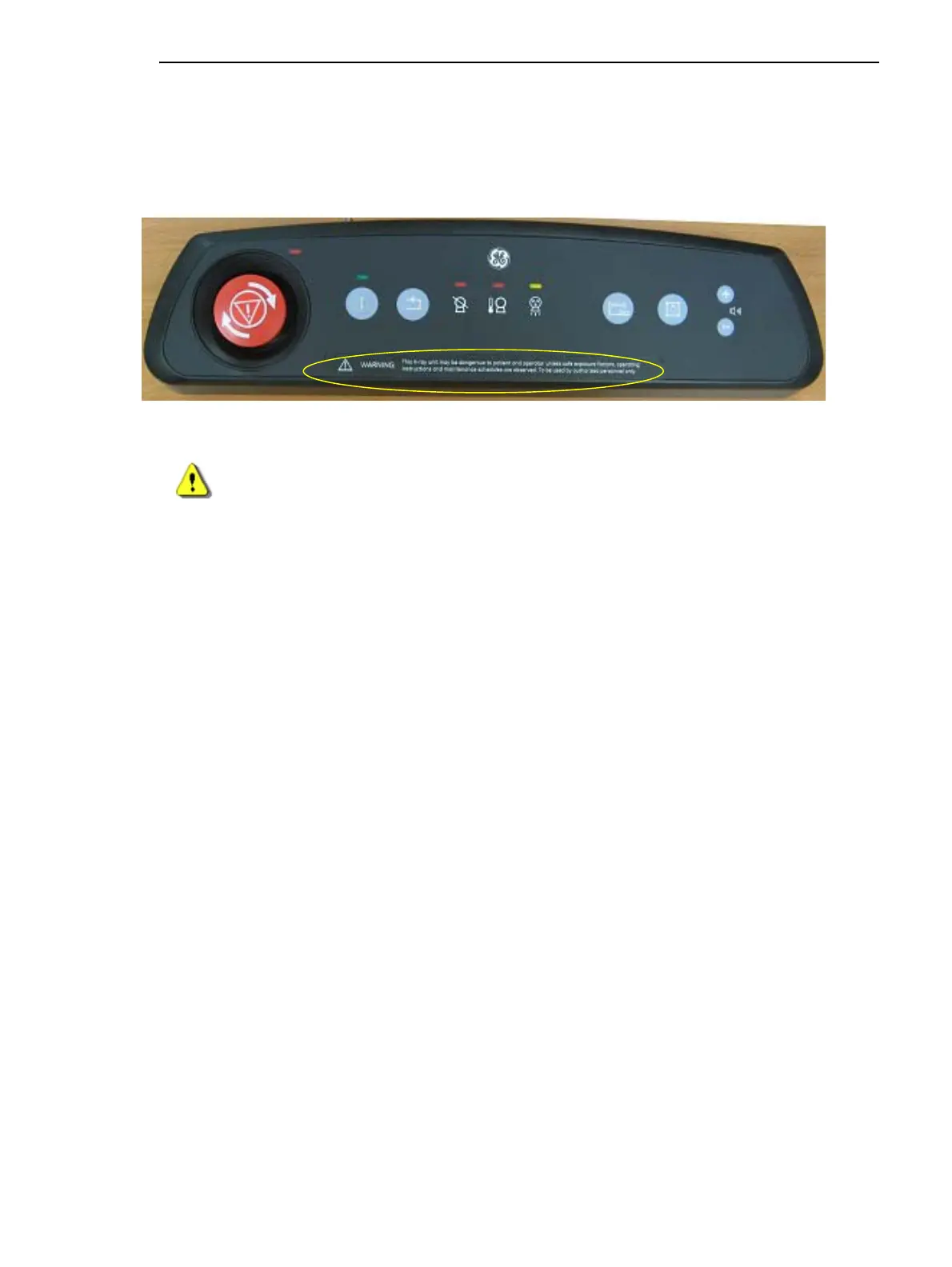
RCIM Label
Figure 2-13 RCIM Label
WARNING This X-Ray unit may be dangerous to the patient and operator, unless
safe exposure factors, operating instructions and maintenance sched-
ules are observed. To be used by authorized personnel only.
UDI Label
Every Optima XR646 system has an unique marking for identification. The Unique Device
Identification (UDI) marking appears on the product label which is located on system cabinet.
UDI: Unique Device Identifier - A UDI is an unique numeric or alphanumeric identification code
assigned to medical devices by the manufacturer of the device. An unique device identifica-
tion marking is applied to a Product Model that is designated as a medical device as per FDA
UDI regulation.
 Loading...
Loading...