Probes and Biopsy
5-18 LOGIQ V2/LOGIQ V1 – User Guide
Direction 5610736-100
Rev. 9
Planned Maintenance
The following maintenance schedule is suggested for the
system and probes to ensure optimum operation and safety.
Returning/Shipping Probes and Repair Parts
US Department of Transportation and GE policy requires that
equipment returned for service MUST be clean and free of blood
and other infectious substances.
When you return a probe or part for service (Field Engineer or
customer), you need to clean and disinfect the probe or part
prior to packing and shipping the equipment.
Ensure that you follow probe cleaning and disinfection
instructions provided in the Basic User Manual.
This ensures that employees in the transportation industry as
well as the people who receive the package are protected from
any risk.
Sterile Ultrasound Procedures
ONLY ultrasound gel that is labeled as sterile, is sterile.
Ensure you always use sterile ultrasound gel for those
procedures that require sterile ultrasound gel.
Once a container of sterile ultrasound gel is opened, it is no
longer sterile and contamination during subsequent use is
possible.
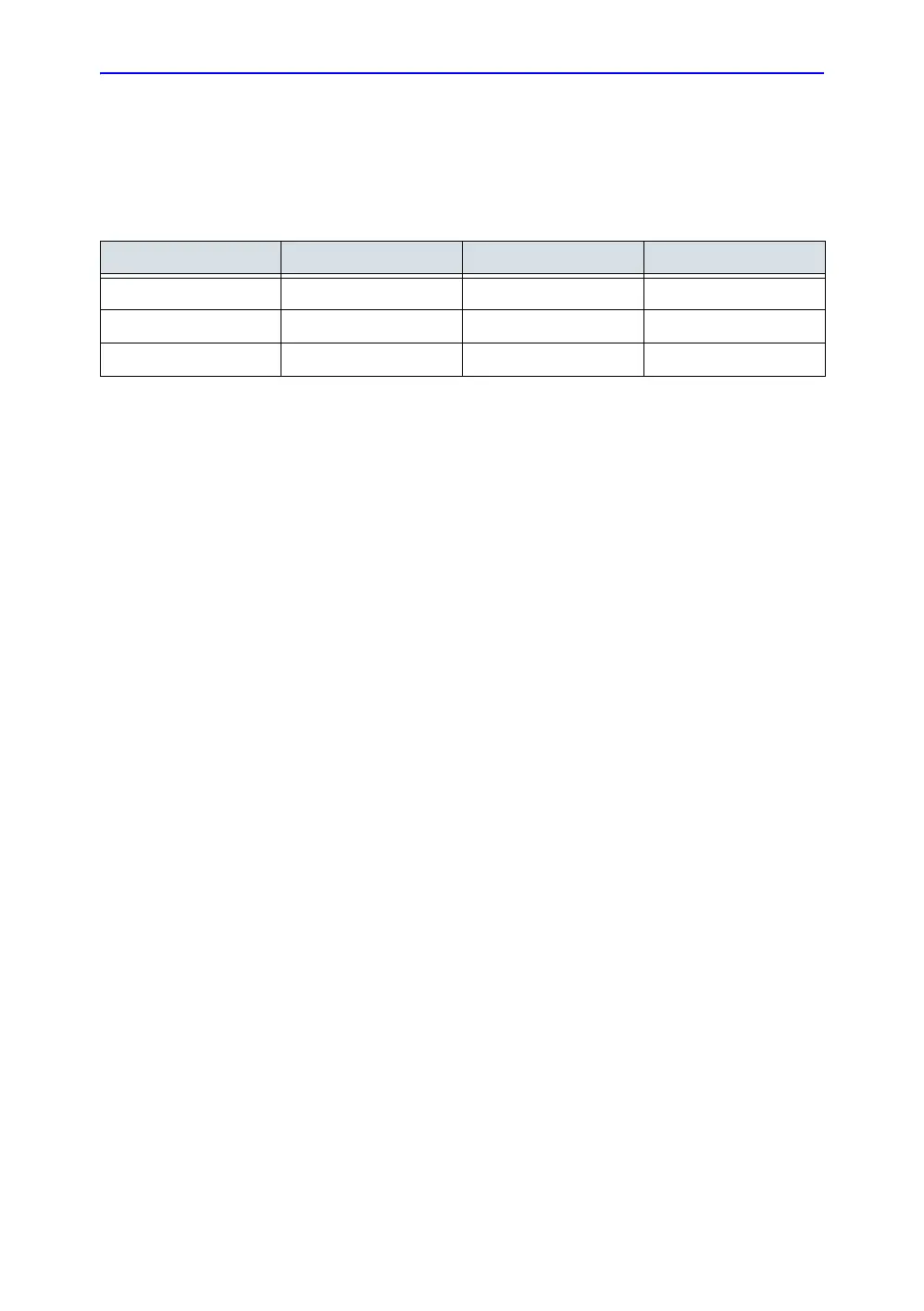
Table 5-5: Planned Maintenance Program
Do the Following Daily After Each Use As Necessary
Inspect the Probes X X X
Clean the Probes X X
Disinfect Probes X X

 Loading...
Loading...