Probes and Biopsy
5-32 LOGIQ V2/LOGIQ V1 – User Guide
Direction 5610736-100
Rev. 9
Fixed Needle Biopsy Guide Assembly
Preparation
To prepare the endocavitary probe for use:
1. Remove the probe from the box and carefully examine it for
any damage.
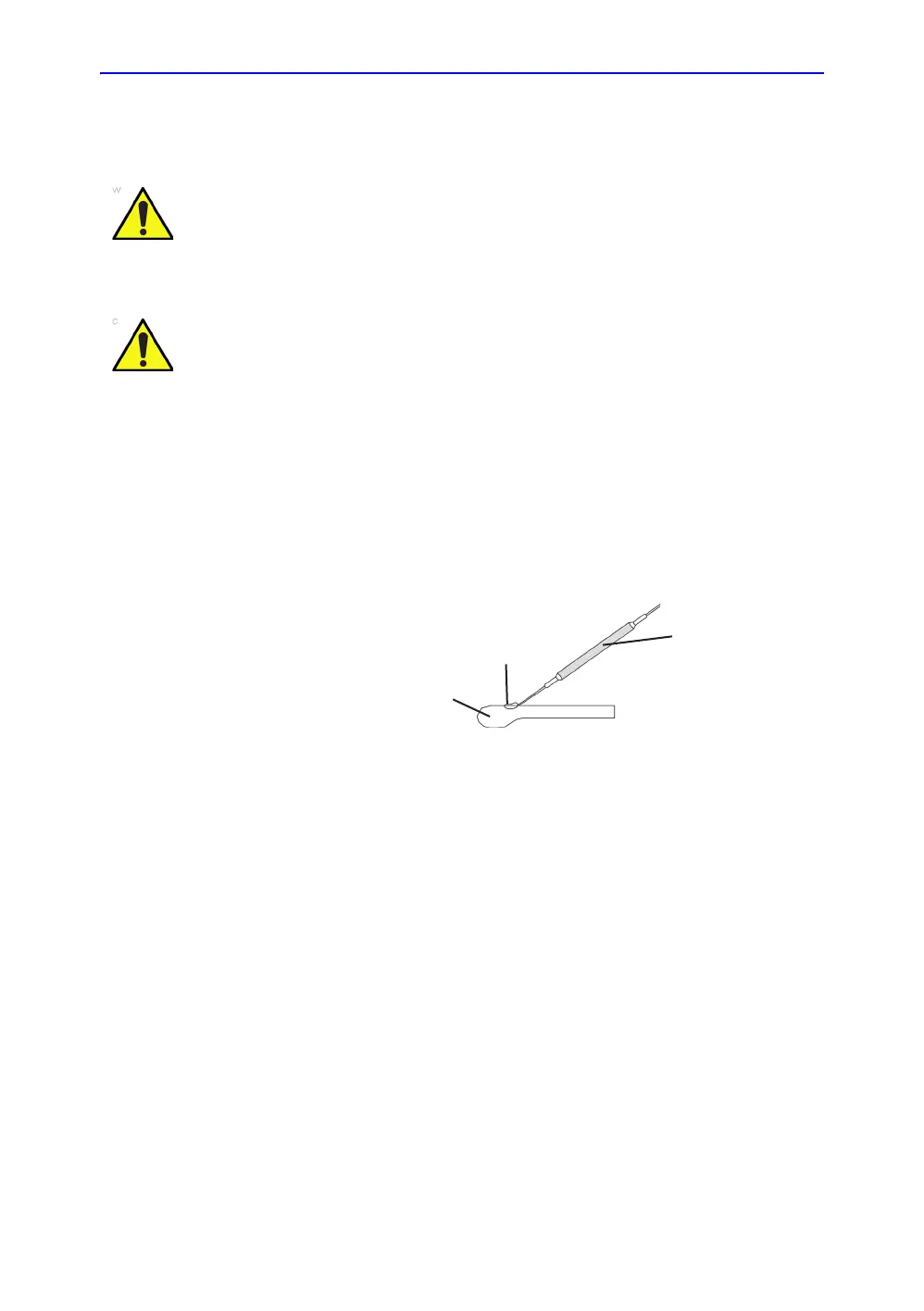
2. If the biopsy guide is to be attached, use the filling removal
tool to clean out the attachment area on the probe head.
Figure 5-5. Attachment Filling Removal
a. Probe Head
b. Attachment
c. Filling Removal Tool
3. Clean, then disinfect the probe.
NOTE: Ensure that protective gloves are worn.
DO NOT use the needle with the catheter (soft tube). There is a
possibility of breaking the catheter in the body.
Before inserting the needle, scan the patient to determine the
correct puncture depth and site. Only the sterile/sanitary
sheath and rubber band are on the probe during the pre-needle
placement scanning.

 Loading...
Loading...