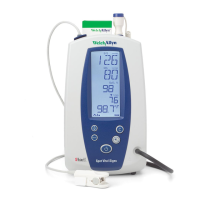
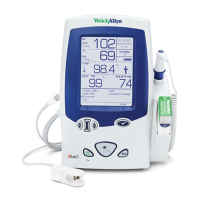
Standards and compliance
General compliance and standards
The monitor complies with the following standards:
21 CFR Subchapter H – Medical Devices – US Food and Drug Administration
2002 No. 236 – Australian Therapeutic Goods Act
93/42/EEC – European Economic Community Medical Devices Directive
2007/47/EC – European Economic Community Medical Devices Directive 2007
Amendment
94/62/EC – European Economic Community Packaging Directive
2002/96/EC – European Economic Community Waste Electrical and Electronic
Equipment Directive
2006/66/EC – European Economic Community Batteries and Accumulators Directive
SOR/98-282 – Canadian Medical Devices Regulation
IATA DGR – International Air Transport Association Dangerous Goods Regulation
United Nations ST/SG/AC.10/11 – Manual of Tests and Criteria, Part III, Sub-Section 38.3
ANSI/AAMI SP10
AS/NZS 3200.1.0
1
ASTM D 4332, E 1104
CAN/CSA C22.2 NO.601.1
1
CAN/CSA-C22.2 NO.60601-1-2, CSA Z9919
EN 1060-1, 1060-3, 1060-4
2
EN/IEC 60601-1, 60601-1-2, 60601-1-8, 60601-2-30, 62304, 80601-2-30, 62366,
60601-1-6
EN/ISO 9919, 13485, 14971, 80601-2-56, 80601-2-61, 81060-2:2013
ISTA 2A
UL 60601-1
1
Directive 2002/96/EC-WEEE:
Disposal of noncontaminated electrical and electronic equipment
This product and its accessories must be disposed of according to local laws and
regulations. Do not dispose of this product as unsorted municipal waste. Prepare this
product for reuse or separate collection as specified by Directive 2002/96/EC of the
European Parliament and the Council of the European Union on Waste Electronic and
1
Standard is essentially the IEC 60601-1 General standard plus the listed country's National Deviations.
2
Non-Invasive Sphygmomanometers – Part 1: General Requirements, Part 3. Supplementary
Requirements for Electro-Mechanical Blood Pressure Measuring Systems, Part 4: Test Procedures to
Determine the Overall System Accuracy of Automated Non-Invasive Sphygmomanometers.
141
 Loading...
Loading...