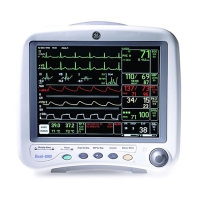
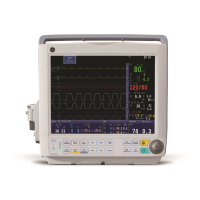
1-16 Dash™ 3000/4000/5000 2000966-386D
Introduction
Underwriters Laboratories, Inc. Classification
FCC Compliance Information Statement
This device complies with Part 15 of the FCC Rules and with RSS-210 of Industry
Canada.
Operation is subject to the following two conditions:
This device may not cause harmful interference, and
This device must accept any interference received, including interference that
may cause undesired operation.
WARNING
—Changes or modifications not expressly approved by the party
responsible for compliance could void the user’s authority to operate
the equipment.
Manual Information
Purpose
This manual contains instructions necessary to operate the monitor safely and in
accordance with its functions and intended use.
Intended Audience
This manual is intended for clinical professionals. Clinical professionals are expected
to have a working knowledge of medical procedures, practices and terminology, as
required for monitoring of critically ill patients.
1
The class of equipment — I or N/A (not applicable).
2
The type of applied part — B, BF, CF, Not Marked or none (no applied parts).
3
Ordinary equipment (enclosed equipment without protection against the ingress of water).
4
Equipment not suitable for use in the presence of a flammable anesthetic mixture with air, oxygen or nitrous oxide.
Medical Equipment
With respect to electric shock, fire and mechanical hazards only in
accordance with UL 60601-1, CAN/CSA C22.2 NO. 601.1, IEC 60601-1,
IEC 60601-2-27, IEC 60601-2-30, IEC 60601-2-34, and IEC 60601-2-49.
 Loading...
Loading...