2 Safety
Dürr Dental has designed and constructed this
unit so that when used properly and for the
intended purpose it does not pose any danger to
people or property. Nevertheless, residual risks
can remain. You should therefore observe the fol-
lowing notes.
2.1 Intended purpose
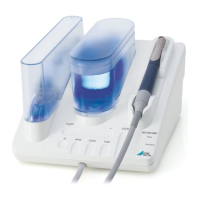
This device is a piezo-operated ultrasonic device
for use in dental applications. It is mainly used for
the treatment of periodontal defects. In addition,
the device is used in the area of prophylaxis, peri-
implantitis treatment as well as dental hygiene.
2.2 Intended use
The ultrasonic device is designed for use in peri-
odontology, for the removal of plaque and for the
cleaning of tooth surfaces. This is done via cavi-
tation, polishing, grinding and scraping. Further
assistance for the treatment can be provided by
using hydroxyl and/or fluorapatite as a polishing
agent in periodontology. Only agents approved
by the manufacturer may be used. The treatment
is gentle on the teeth and almost painless.
Paro handpiece applications
– Periodontal treatment
Thorough removal of biofilm and concrement,
and smoothing of the root surface
– Recall
Removal of biofilm and gentle treatment of root
surfaces even with frequent instrument usage
– Peri-implantitis treatment
Cleaning of implant surfaces using fibrous
composite materials and special plastic instru-
ments. Avoids damaging the implant surfaces
Scaler handpiece applications
– Subgingival and supragingival removal of
dental calculus and concrement
The piezo-ceramic drive of the Vector Scaler
allows efficient removal of deposits while provid-
ing the best and gentlest possible protection of
sensitive tissue structures. The ergonomic design
of the handpiece offers six long-lasting high per-
formance LEDs to provide the best possible illu-
mination even into areas that are difficult to see.
2.3 Improper use
WARNING
Risk of explosion due to ignition of
combustible materials
❯
Do not operate the unit in any rooms in
which inflammable mixtures may be
present, e.g. in operating theatres.
Any other usage or usage beyond this scope is
deemed to be improper. The manufacturer
accepts no liability for damages resulting from
this. In these cases the user/operator will bear
the sole risk.
2.4 General safety information
WARNING
Contraindication
Ultrasound vibrations can interfere with
the function of heart pacemakers and
defibrillators.
❯
Patients with heart pacemakers or
defibrillators should not be treated with
these instruments.
❯
Always comply with the specifications of all
guidelines, laws, and other rules and regula-
tions applicable at the site of operation for the
operation of this unit.
❯
Check the function and condition of the unit
prior to every use.
❯
Do not convert or modify the unit.
❯
Comply with the specifications of the Installa-
tion and Operating Instructions.
❯
The Installation and Operating Instructions
must be accessible to all operators of the unit
at all times.
2.5 Specialist personnel
Operation
Unit operating personnel must ensure safe and
correct handling based on their training and
knowledge.
❯
Instruct or have every user instructed in han-
dling the unit.
Important information
9000-615-28L02 1903V004 5
EN
 Loading...
Loading...











