Probes and Biopsy
5-40 LOGIQ V2/LOGIQ V1 – User Guide
Direction 5610736-100
Rev. 9
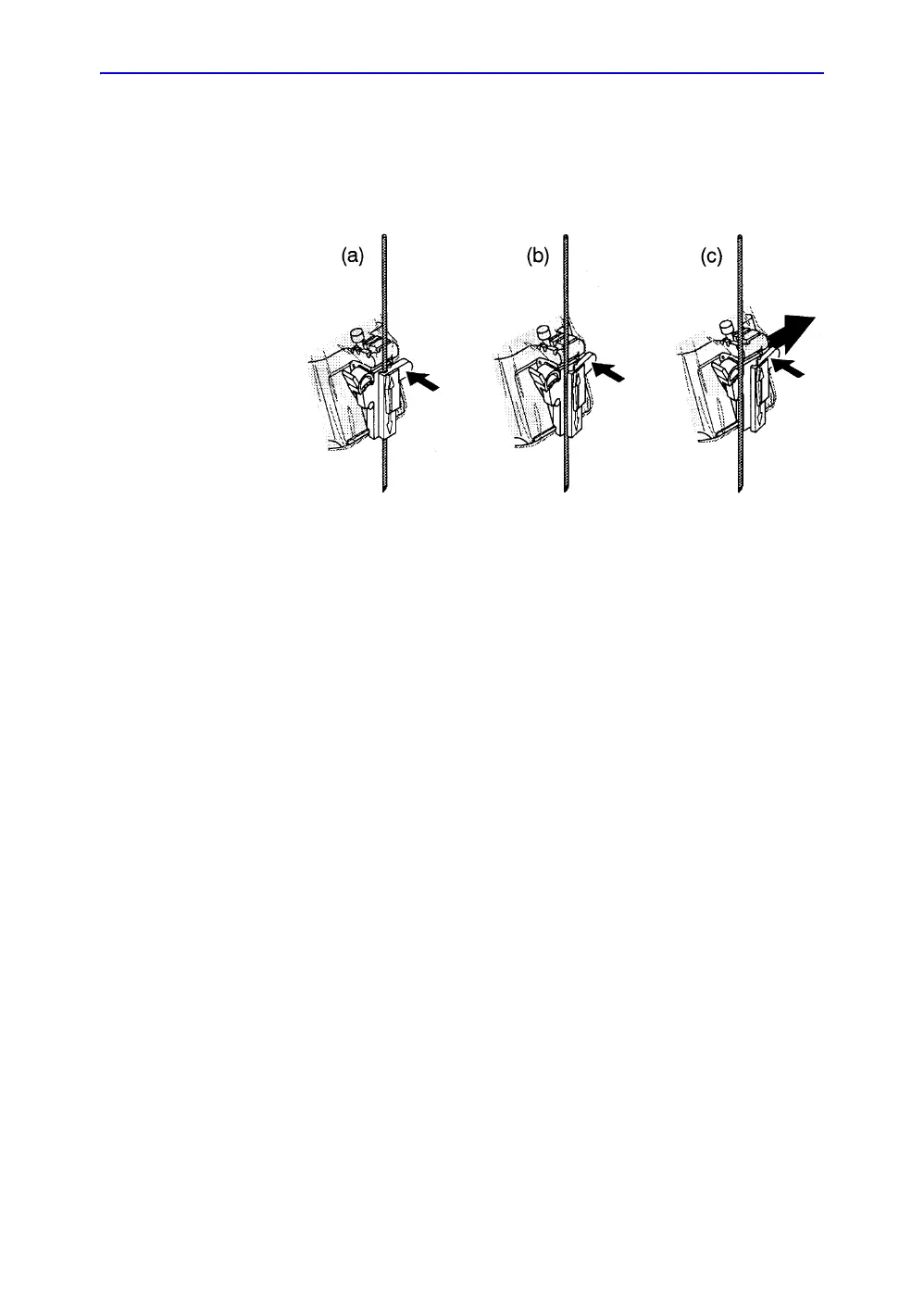
Releasing the needle
According to the following procedure, you remove the needle
from a probe and an assembly without moving the needle.
Figure 5-19. Release the needle from assembly
a. Push the knob portion of a sleeve in the direction of the
arrow.
b. The needle is released from the assembly.
c. Push the probe and the assembly in the direction of the
larger arrow to remove the needle.
Biopsy Needle Path Verification
To verify that the path of the needle is accurately indicated within
the guidezone on the system monitor, perform the following:
• Properly install the bracket and biopsy guide.
• Scan in a container filled with water (47° C).
• Display the biopsy guidezone on the monitor.
• Ensure that the needle echo falls within the guidezone
markers.
 Loading...
Loading...