D9U001MCX-0101_03
21
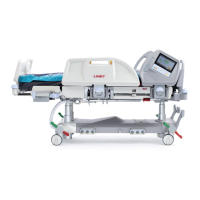
3 Intended use
The intended use is the hospitalization of the patient in the intensive and acute care units, which includes above all the
following aspects:
► Adjustment of the specic positions needed for the preventive reasons, routine nursing, treatments, mobilization, physio-
therapy, examinations, sleeping, and relaxation. These positions are further specied and described in the clinical evaluation of this
device, together with their potential clinical outcomes and benets.
► Providing the safe environment for the patient during all relevant procedures. The particular requirements on patient safety
are the subject of the clinical evaluation, including evaluation of the risk/benet ratio. The relevant safety issues are the part of the
risk management le.
► Patient in-bed indoor transport out of the patient room.
► Providing the suitable working conditions for the caregivers to perform the routine and specic tasks during the patient
hospitalization.
► Indicative measurement of the patient weight, used as supportive feature without direct diagnostic eect. It helps sta to
assess the general patient status and apply the nutrition and medicaments (valid for the version of the beds with in-bed scales).
3.1 User population
► Adult patients (weight >= 40 kg, height >= 146 cm, BMI >= 17) in the intensive and acute care units (Application Environ-
ment 1 and 2 as in IEC 60601-2-52)
► Caregivers (nurses, doctors, technical personnel, transport personnel, cleaning personnel)
3.2 Contraindications
► The medical device is not intended for the pediatric patients use.
► Certain positions are not suitable for specic diagnoses/medical conditions (e.g. spinal cord injuries vs. Fowler position,
higher ICP patients vs. Trendelenburg). Sta expert assessment / nursing consideration is needed in all individual case of contrain-
dication.
3.3 Operator
► Caregiver
► Patient (based on individual patient status assessment by caregiver the patient can utilize dedicated device functions)
 Loading...
Loading...