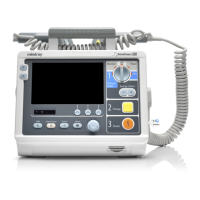
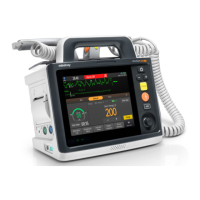
Defibrillator/Monitor Operator’s Manual 25 - 1
25 Accessories
The accessory material that contacts the patients has undertaken the bio-compatibility test and is verified to be
in compliance with ISO 10993-1.
WARN ING
• Use accessories specified in this chapter. Using other accessories may cause damage to the
equipment or not meet the claimed specifications.
• Single-use accessories are not designed to be reused. Reuse may cause a risk of contamination and
affect the measurement accuracy.
• Check the accessories and their packages for any sign of damage. Do not use them if any damage is
detected.
• At the end of its service life, the equipment, as well as its accessories, must be disposed of in
compliance with the guidelines regulating the disposal of such products to avoid contaminating the
environment.
• When using the accessories, consider the accessories’ operating temperature. Refer to
corresponding accessory’s instruction for use for details.
25.1 ECG Accessories
25.1.1 ECG Electrodes
25.1.2 12-pin Trunk Cable
Model Specification Applicable patient PN
31499224 10 pcs/pack Adult 0010-10-12304
2245 50 pcs/pack Pediatric 9000-10-07469
2258-3 3 pcs/pack Neonate 900E-10-04880
Leadwire
supported
Model Compatible
with
Type Applicable
patient
PN
3-lead EV 6202 AHA, IEC Defibrillation-proof Pediatric,
neonate
0010-30-42720
3/5-lead EV 6201 AHA, IEC Defibrillation-proof Adult, pediatric 0010-30-42719

 Loading...
Loading...