Regulatory requirement
This product complies with regulatory requirements of the following European
Directive 93/42/EEC concerning medical devices.
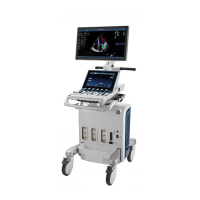
This manual is a reference for the Vivid S70 and Vivid S60 ultrasound systems and
covers the following models: Vivid S70 v203 and Vivid S60 v203. It applies to all
revisions of the 203 software for the Vivid S70 and Vivid S60 ultrasound systems,
which will hereafter be listed as Vivid S70 / S60. All information in this manual is
relevant for the two systems unless otherwise specified.
© GE Medical Systems. All rights reserved. No part of this manual may
be reproduced, stored in a retrieval system, or transmitted, in any form
or by any means, electronic, mechanical, photocopying, recording, or
otherwise, without the prior written permission of GE Medical Systems.
COMPANY DATA GE Medical Systems, Israel Ltd.
Nativ Ha’or Street no.1
3508510, Haifa
ISRAEL
Tel: (+972) 4 8519 555 Fax: (+972) 4 8519 500
GE Medical Systems SCS
283 rue de la Minière, 78530 BUC, France
 Loading...
Loading...