Vivid S70 / S60 – User Manual i-3
BC092760-1EN 01
Certifications
• Quality management standards for medical devices: GE
Medical Systems, Israel Ltd. and GE Medical Systems,
China Co. Ltd. are ISO13485 certified.
Importer Information
• TURKEY
• BRAZIL
Directives
The GE ultrasound product families are tested to meet all
applicable requirements in relevant EU Directives and
European/International standards.
• Council Directive 93/42/EEC concerning MDD (Medical
Devices Directive): the CE label affixed to the product
testifies compliance to this Directive.
The location of the CE marking is specified in ‘Device labels’
on page 2-26.
• Year of first CE mark: 2015
Classifications
According to 93/42/EEC Medical Device Directive, this is a
Class IIa Medical Device.
The following classifications are in accordance with the IEC/
EN 60601-1:
• According to IEC/EN 60601-1, Equipment is Class I, with BF
or CF Applied Parts.
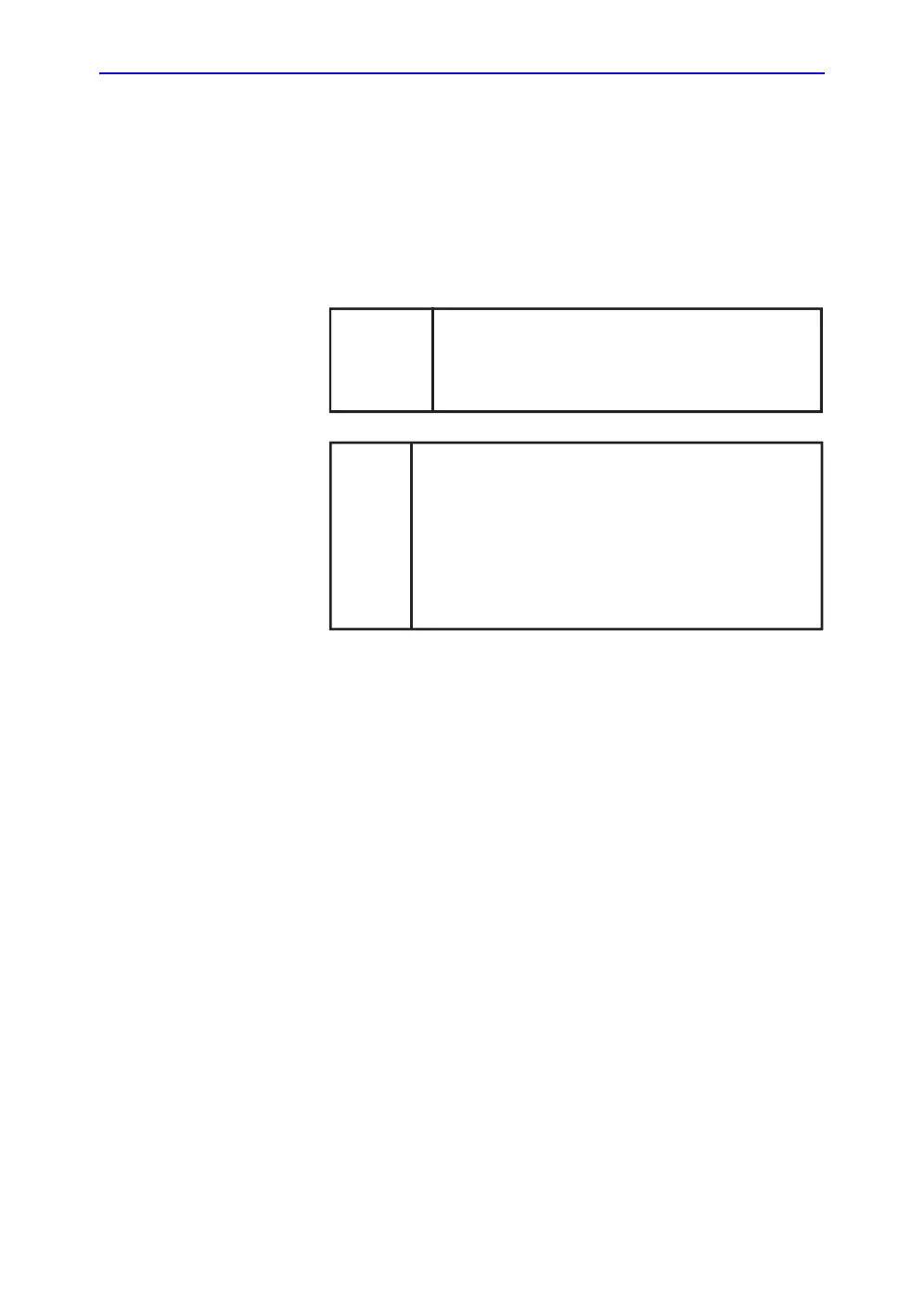
GE Medical Systems Türkiye Ltd. Şti.
Esentepe Mah. Harman Sok. No: 8
34394 Şişli İstanbul Türkiye
Türkiye
İthalatçısı /
Turkish
Importer
GE Healthcare do Brasil Comércio e Serviços
para Equipamentos Médico- Hospitalares Ltda
Av. Magalhães de Castro, 4800
Andar 11 Conj. 111 e 112, Andar 12 Conj. 121 e
122, Torre 3 - Cidade Jardim
São Paulo SP – CEP: 05676-120
C.N.P.J.: 00.029.372/0001-40
Número de Registro ANVISA: 80071260360
Brazilian
Importer
 Loading...
Loading...