g
GE Medical Systems
Regulatory Requirements
This product complies with regulatory requirements of the
following European Directive 93/42/EEC concerning medical
devices.
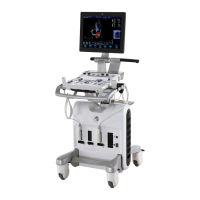
This manual is a reference for the Vivid S5 and Vivid S6. It
applies to all versions of the 10.2.x software for the Vivid S5
and Vivid S6 ultrasound systems.
PRELIMINARY
MANUAL STATUS
R2424458-100
07 February 2010
Doc ID: DOC0600341
GE Medical Systems. All rights reserved. No part of this
manual may be reproduced, stored in a retrieval system, or
transmitted, in any form or by any means, electronic,
mechanical, photocopying, recording, or otherwise,
without the prior written permission of GE Medical
Systems.
COMPANY DATA GE Medical Systems, Israel Ltd.
4 Etgar Street
39120 Tirat Carmel
Israel
Tel: (+972) 4851 9555 Fax: (+972) 4851 9500
GE Medical Systems Information Technologies GmbH,
Munzinger Strasse 5 D-79111 Freiburg, Germany
Tel: (+49) 761 45 43 0 Fax: (+49) 76145 43 233
 Loading...
Loading...