2B-2 - ELECTRICAL 90-830234R3 DECEMBER 1997
Special Tools
1. Volt/Ohm/DVA Meter 91-99750
2. Hydrometer (obtain locally)
3. Ammeter (obtain locally)
Battery
Precautions
When charging batteries, an explosive gas mixture
forms in each cell. A portion of this gas escapes thru
holes in vent plugs and may form an explosive atmo-
sphere around battery if ventilation is poor. This ex-
plosive gas may remain in or around battery for sev-
eral hours after it has been charged. Sparks or
flames can ignite this gas and cause an internal ex-
plosion which may shatter the battery.
The following precautions should be observed to pre-
vent an explosion.
1. DO NOT smoke near batteries being charged or
which have been charged very recently.
2. DO NOT break live circuits at terminals of batter-
ies because a spark usually occurs at the point
where a live circuit is broken. Always be careful
when connecting or disconnecting cable clamps
on chargers. Poor connections are a common
cause of electrical arcs which cause explosions.
3. DO NOT reverse polarity of battery cables on bat-
tery terminals.
CAUTION
If battery acid comes into contact with skin or
eyes, wash skin immediately with a mild soap.
Flush eyes with water immediately and see a
doctor.
Operating Engine Without Battery
If desired (or in an emergency), engines equipped
with an alternator can be started and operated with-
out a battery (either disconnected or removed) if
“Warning”, below, is followed.
WARNING
Before operating engine with battery leads dis-
connected from battery, disconnect stator leads
(YELLOW) from rectifier. Insulate (tape) stator
lead ring terminals.
Specific Gravity Readings
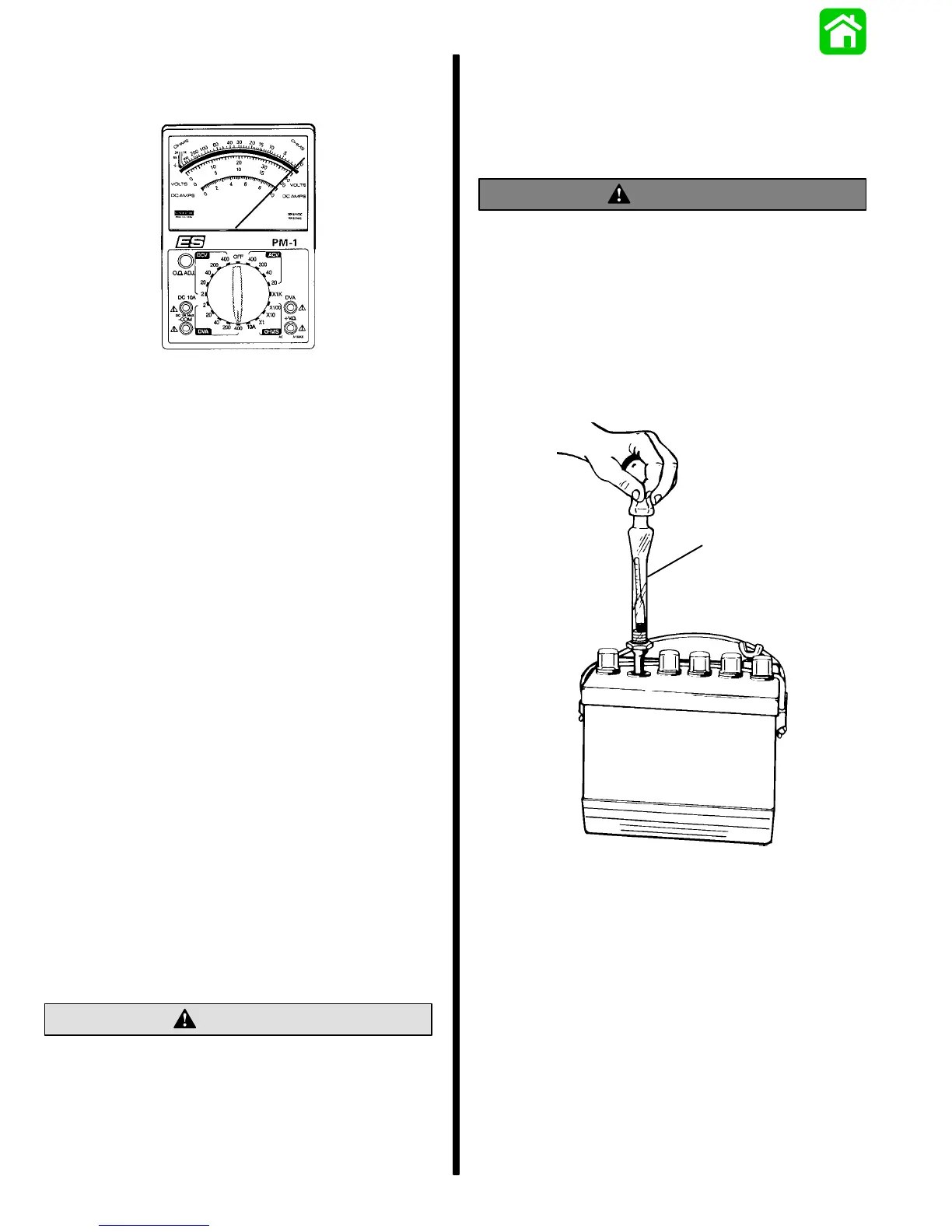
Use a hydrometer to measure specific gravity of elec-
trolyte in each cell.
22532
a
a - Hydrometer
Hydrometer measures percentage of sulphuric acid
in battery electrolyte in terms of specific gravity. As a
battery drops from a charged to a discharged condi-
tion, acid leaves the solution and chemically com-
bines with the plates, causing a decrease in specific
gravity of electrolyte. An indication of concentration
of electrolyte is obtained with a hydrometer.
When using a hydrometer, observe the following
points:
1. Hydrometer must be clean (inside and out) to in-
sure an accurate reading.

 Loading...
Loading...
















