Draeger Medical Systems, Inc.
16 Electronics Avenue
Danvers, MA 01923
USA
Authorized EC representative:
Dräger Medical AG & Co. KGaA
Moislinger Allee 53-55
23558 Lübeck
Germany
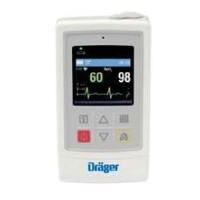
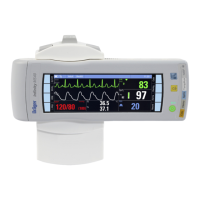
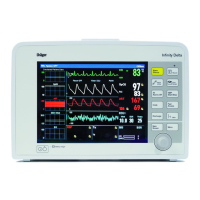
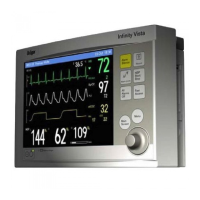
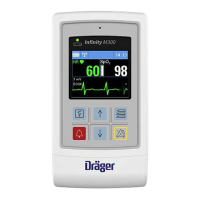
Infinity Gamma Series User’s Guide
Software Version VF4
This product is covered by one or more of the following patents: 5,224,484;
5,224,740; 5,240,008; 5,285,791; 5,355,890; 5,337,751; 5,375,604.
This device bears the ! label in accordance with the provisions of the Direc-
tive 93/42/EEC of June 14, 1993 concerning medical devices.
!"
0123
Dräger Medical AG & Co. KGaA, 2003. All rights reserved.
Printed in the United States of America.
Dräger reserves the right to modify the design and specifications contained
herein without prior notice. Please contact your local Dräger Sales Represen-
tative for the most current information.
Reproduction in any manner, in whole or in part, in English or in any other
languages, except for brief excerpts in reviews and scientific papers, is pro-
hibited without prior written permission of Dräger Medical AG & Co. KGaA.
All Dräger devices are intended for use by qualified medical personnel only.
CAUTION: Federal Law in the United States restricts these devices to
sale by, or on order of a physician.
Before using all Dräger devices, read all the manuals that are provided with
your device carefully. Patient monitoring equipment, however sophisticated,
should never be used as a substitute for the human care, attention, and critical
judgment that only trained health care professionals can provide.

 Loading...
Loading...