Specifications
Instructions for Use Apollo SW 4.5n 303
Part Number: 9053586, 3rd edition
EMC declaration
General information
The EMC compliance of the product has been evaluated
with the external cables, transducers, and accessories
specified in the list of accessories. Other accessories which
do not affect EMC compliance may be used if no other rea-
sons forbid their use (see other sections of the instructions
for use). The use of noncompliant accessories can result in
increased emissions or decreased immunity of the medical
device.
The medical device may only be used adjacent to or
stacked with other devices when the configuration is ap-
proved by Dräger. When use adjacent to or stacked with
other devices is absolutely necessary without the configura-
tion being approved by Dräger, the correct operation of the
device in this configuration must be tested before the prod-
uct is used. In any case, strictly observe the instructions for
use of the other devices.
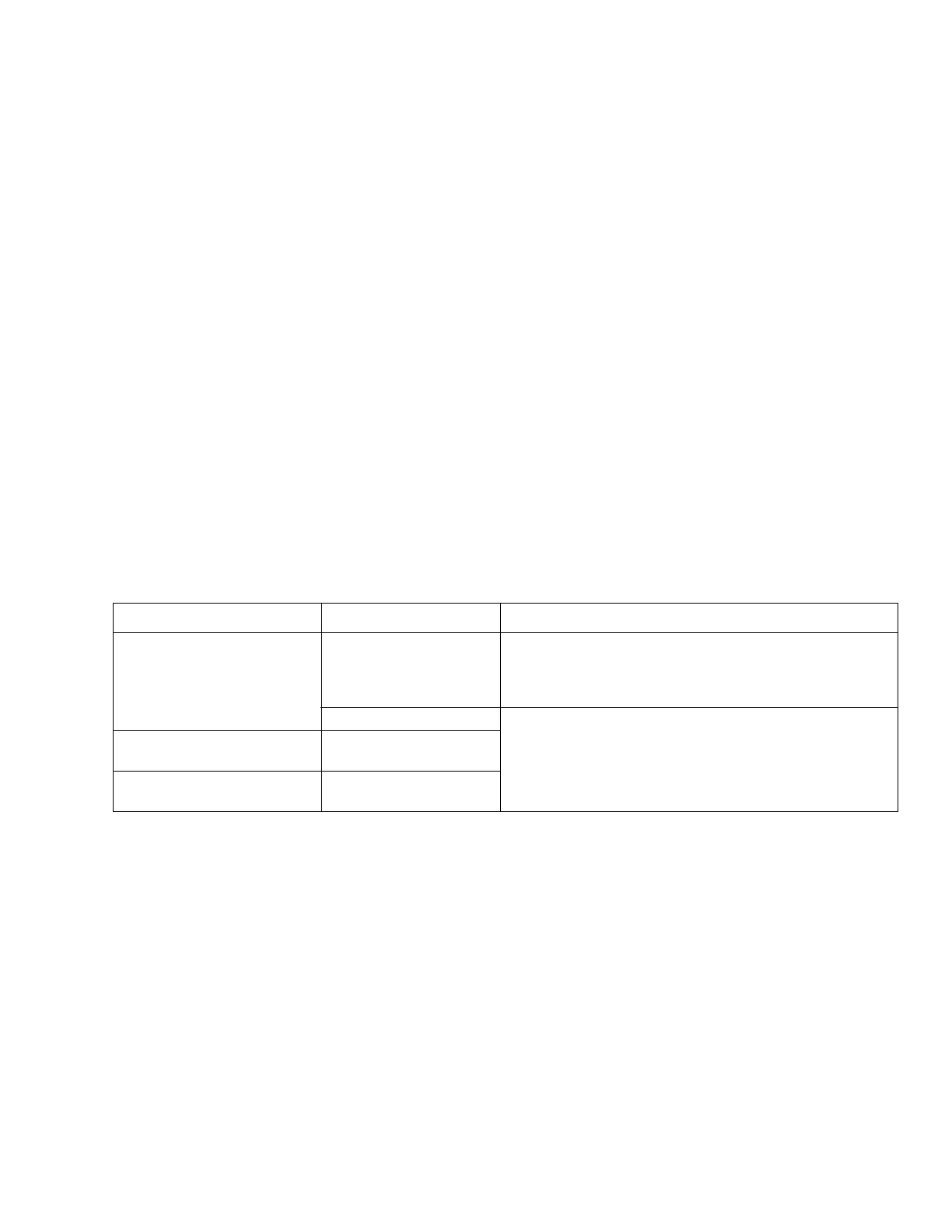
Electromagnetic emissions
Electromagnetic environment
The medical device is intended for use in an electromag-
netic environment as specified below. The user must ensure
that the medical device is used in such an environment.
Emissions Compliance according to Electromagnetic environment
RF emissions (CISPR 11) Group 1 The medical device uses radio frequency energy only for its
internal function. Therefore, its radio frequency emissions are
very low and are not likely to cause any interference in nearby
electronic equipment.
Class A The medical device is suitable for use in all establishments
other than domestic establishments and those directly
connected (without transformer) to the public low-voltage
power supply network that supplies buildings used for domestic
purposes.
Harmonic emissions
(IEC 61000-3-2)
Not applicable
Voltage fluctuations / flicker
(IEC 61000-3-3)
Not applicable

 Loading...
Loading...