20-12 Art: 714382-00D Rev. Date: 02/20/06
Operator Sample
Handling/Cartridge
Verification When Verified
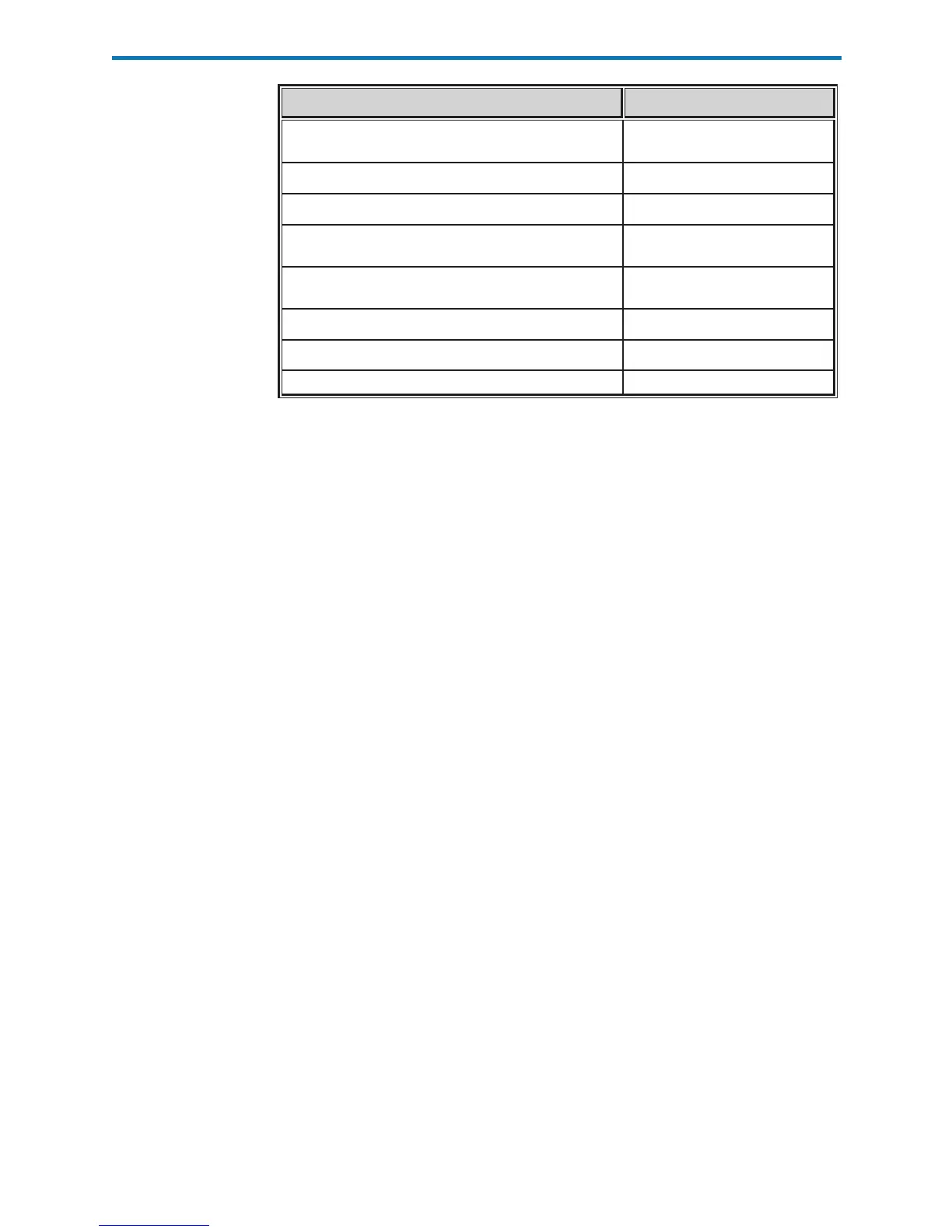
Verify the cartridge inserted has not been previously used Every cartridge use
Verify the calibrant pack has not prematurely ruptured Every cartridge use
Verify the electronic contact pads are dry and uncontaminated Every cartridge use
Verify the proper amount of sample was placed into the sample
chamber
Every cartridge use
Verify the sample was properly positioned within the sample
chamber
Every cartridge use
Verify the sample is free of included bubbles Every cartridge use
Verify the sample is not clotted Every cartridge use
Verify the sample chamber is properly sealed with the snap closure Every cartridge use
Validating the
Performance of the
i-STAT System
Until recently, regulations and laboratory accreditation standards specified the
use of traditional quality control regimens, including the daily use of liquid
“control” materials.
As new technologies such as the i-STAT System have become available, the
community has recognized the limitations of relying upon traditional regimens,
prompting various regulatory and accreditation organizations to modify their
standards accordingly.
Many of the newly drafted regulations and accreditation standards recognize
the danger of denoting specific methods of achieving an effective quality
control regimen. Additionally, specific methods cannot anticipate future
technological changes, so many of the regulatory and accreditation
organizations are changing their standards to place the responsibility of
establishing and validating the quality system a laboratory employs on the
laboratory director.
Quality control regimens should be established using information from the
manufacturer and scientific literature.
It is important to validate the performance of the i-STAT System and the
recommended quality control regimen to develop personal confidence in
our approach to the challenges of putting a diagnostic device in the hands of
individuals untrained in laboratory science.
Some of the regulatory and accreditation organizations recommend the daily
use of liquid “control” materials for the first month of use, slowly stepping back
the frequency as a database of performance information increases confidence
levels. The number of lots of materials examined should also be considered
when determining a validation protocol.

 Loading...
Loading...