Abbott Point of Care Inc. • Abbott Park, IL 60064 • USA
Art: 730271-00A Rev. Date: 05-Mar-12
TECHNICAL BULLETIN
i-STAT
®
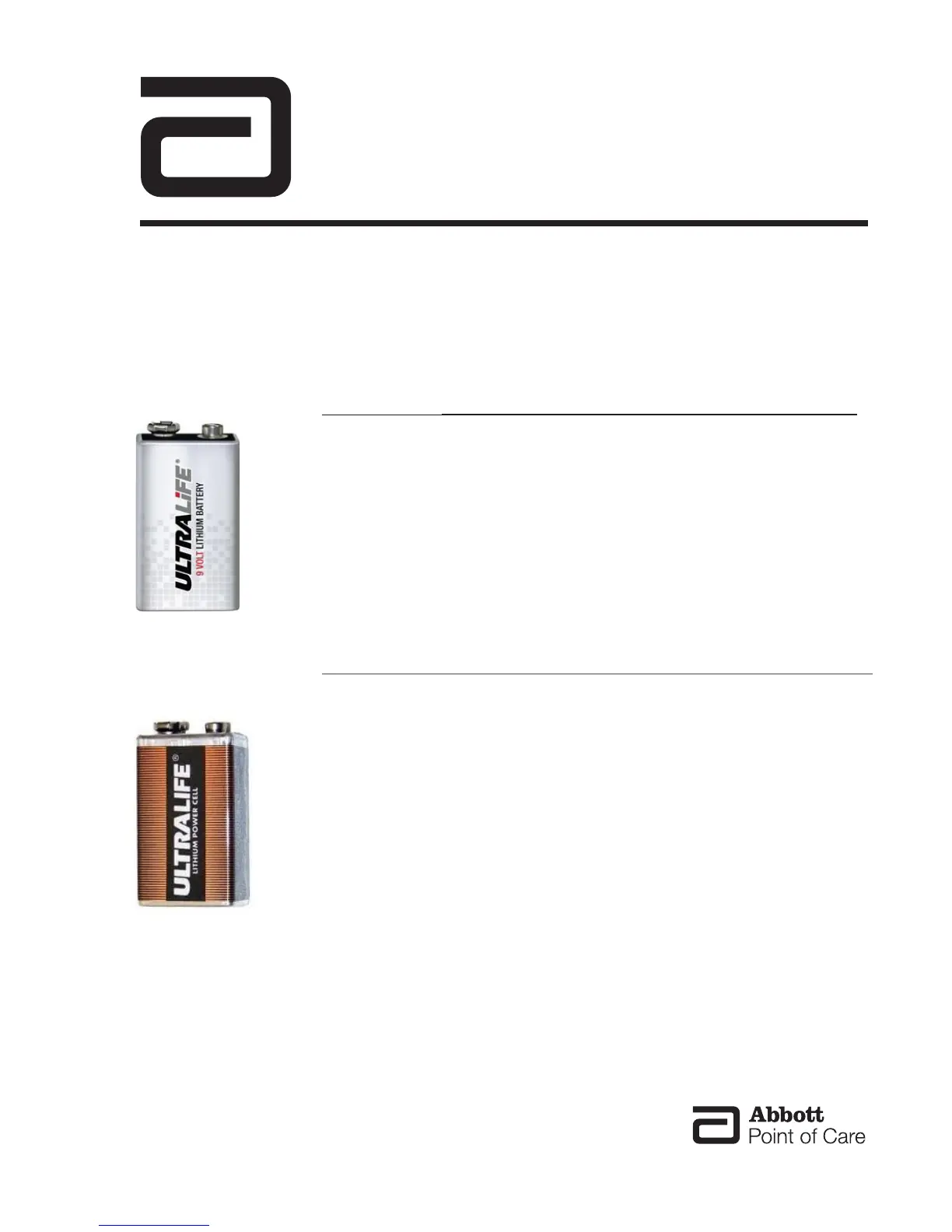
NEW
Ultralife 9-Volt
Lithium Battery
CURRENT
Ultralife 9-Volt
Lithium Battery
NEW ULTRALIFE 9-VOLT LITHIUM BATTERY
FOR USE WITH THE i-STAT SYSTEM
i-STAT is a registered trademark of the Abbott Group of Companies in various jurisdictions.
1. OVERVIEW
Ultralife Battery and Energy Products, the manufacturer of the current 9-volt
lithium battery (Part No. U9VL-J) sold by Abbott Point of Care (APOC) for use with
the i-STAT System is releasing a new 9-volt lithium battery (Part No. U9VL-J-P),
which will replace the current U9VL-J battery for shipment to i-STAT customers.
This Technical Bulletin describes general notes and considerations regarding
ordering and use of the new Ultralife 9-volt lithium battery.
Note: The new Ultralife 9-volt lithium battery has a safety feature that provides
protection preventing the i-STAT handheld from overheating due to component
failure within the analyzer circuitry.
2. GENERAL NOTES AND CONSIDERATIONS
1. The new Ultralife 9-volt lithium battery has the same Abbott List Number as
the current battery – 06F21-26. The new battery will also be packaged the same
as the current battery, with each box containing 6 batteries.
2. The new batteries are installed and removed using the same procedure as
the current battery. Instructions for removal and replacement can be found in
Section 17 of the i-STAT 1 System Manual: Routine Care of the Analyzer and
Downloader or Section 2 of the i-STAT System Manual: Portable Clinical
Analyzer.
3. The capacity for the new Ultralife 9-volt lithium battery is the same as the
current battery. Information regarding the expected lifetime for a pair of lithium
batteries can be found in the Disposable Batteries paragraph in Section 2 of
the i-STAT 1 System Manual: i-STAT 1 Analyzer. or in the Power paragraph in
Section 2 of the i-STAT System Manual: Portable Clinical Analyzer.

 Loading...
Loading...