Rev. Date: 11-Jul-11 Art: 714173-00M NA - 3
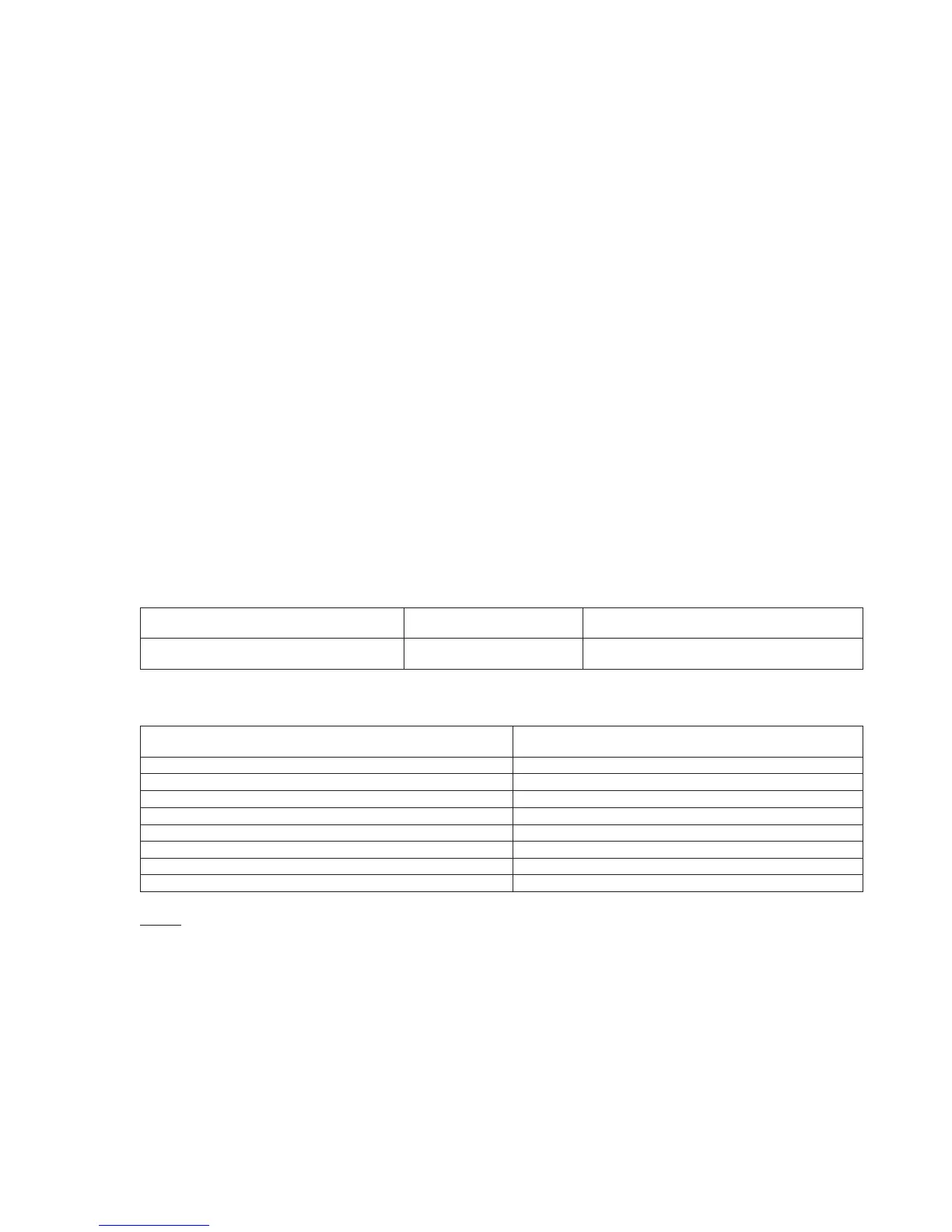
Slope 1.00 0.98 0.95
Int’t -0.11 3.57 5.26
Sy.x 1.17 1.04 1.53
Xmin 126 120 124
Xmax 148 148 148
r 0.865 0.937 0.838
Cartridge Comparison
The performance characteristics of the sensors are equivalent in all cartridge configurations. System
difference analysis was performed on 40 patient samples using the i-STAT 6+ and i-STAT EC4+
cartridges. In the 130–150 mmol/L range the average difference was 0.750.
Factors Affecting Results*
Sodium heparin may increase sodium results up to 1mmol/L
7
.
Hemodilution of the plasma by more than 20% associated with priming cardiopulmonary bypass
pumps, plasma volume expansion or other fluid administration therapies using certain solutions
may cause clinically significant error on sodium, chloride, ionized calcium and pH results. These
errors are associated with solutions that do not match the ionic characteristics of plasma. To avoid
these errors when hemodiluting by more than 20%, use physiologically balanced multi-electrolyte
solutions containing low-mobility anions (e.g. gluconate) such as Normosol
®
-R (Abbott Laboratories),
Plasma-Lyte
®
-A (Baxter Healthcare Corporation), and Isolyte
®
-S (B Braun Medical) rather than solutions
such as normal saline or Ringer’s Lactate.
Test concentrations used were as per the CLSI guidance document,
8
unless otherwise indicated.
The following substances are known to interfere with the i-STAT sodium assay:
Substance
Test Concentration
(mmol/L)
Interference
Bromide 37.5
Increased i-STAT Sodium results. See
Note below.
The following substances are known not to significantly interfere with the i-STAT sodium assay at
the stated test concentrations:
Substance
Test Concentration
(mmol/L)
Acetaminophen 1.32
Acetylcysteine 10.2
Ascorbate 0.34
Bromide (therapeutic) 2.5
9,10,11
β-Hydroxybuterate 6.0
12
Lactate 6.6
Magnesium Chloride 1.0
Salicylate 4.34
Note:
1) Bromide has been tested at two levels; the CLSI recommended level and a therapeutic plasma
concentration level of 2.5 mmol/L. The latter is the peak plasma concentration associated with halothane
anesthesia, in which bromide is released. APOC has not identified a therapeutic condition that would
lead to levels consistent with the CLSI recommended level. Bromide at a concentration of 37.5 mmol/L
increased i-STAT sodium results, while a therapeutic range of bromide (2.5 mmol/L) did not significantly
interfere with i-STAT sodium results.
*It is possible that other interfering substances may be encountered. The degree of interference at concentrations other than those listed might not
be predictable.

 Loading...
Loading...