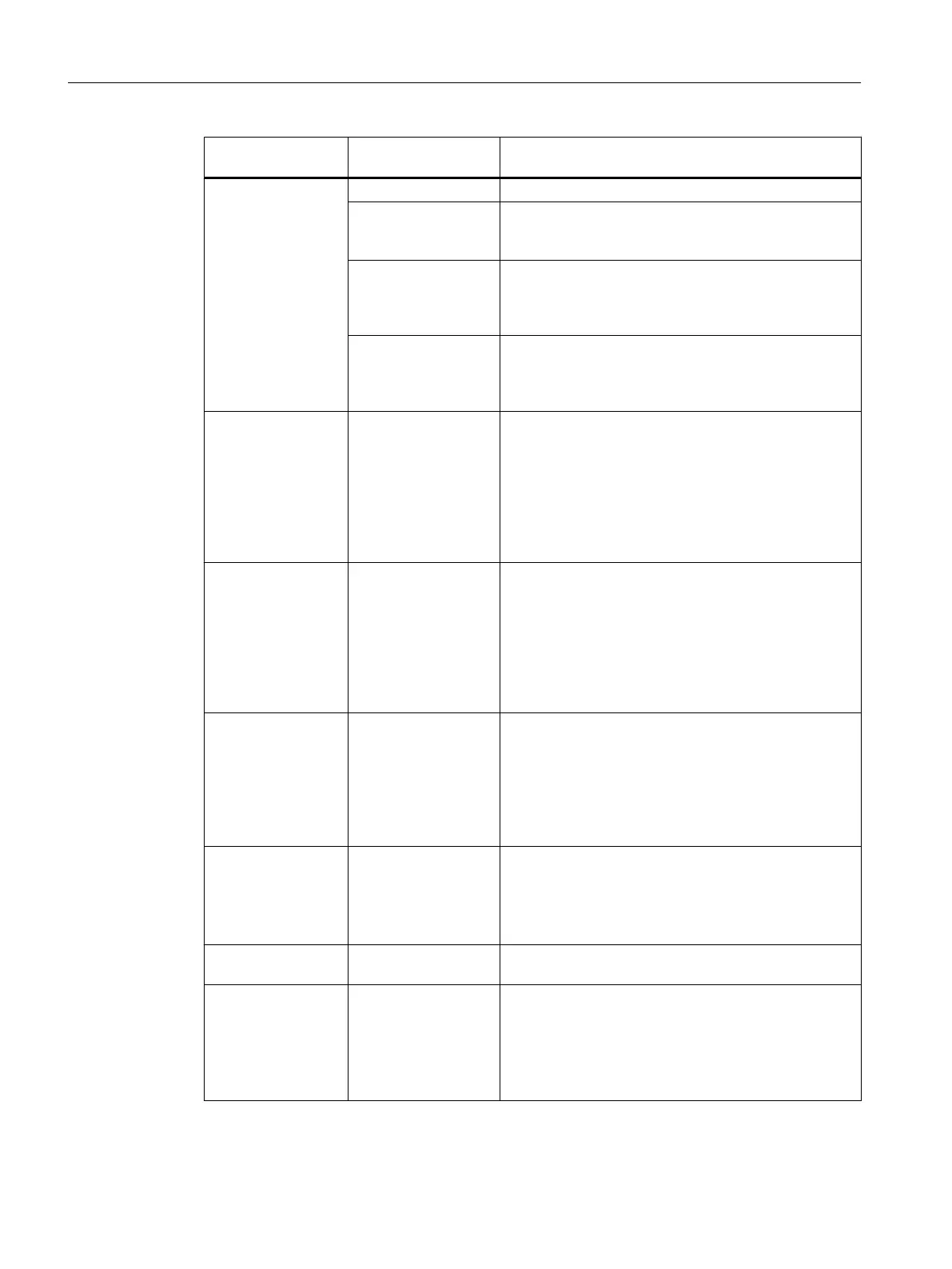
Tab What is set or dis‐
played
Meaning/comment
Product Product, Quality Settings for the main product
Product code Unique numeric code for the project (e.g. an internal
command code) with which the product can be identi‐
fied.
Reference quantity The reference quantity is meant for quantity scaling, this
means, the parameters in the recipe relate to this value
and they have to be adjusted in the event of discrepan‐
cies in the batch quantity.
Minimum scale, maxi‐
mum scale
Limits the upper and lower limits of the batch quantity.
In batch planning, the quantities specified are checked
to make sure they are not lower/higher than the values
entered here.
Input material Material list For the steps linked to EOPs, EPHs or the operator di‐
alogs, the entries are taken from the basic automation;
for a library reference from the library operation. New
materials can also be created. For each input material
configured, the material, quality, scaling function and
required quantity are specified.
Either concrete values (internal formula) or reference to
values of an external formula.
Output material Material list For the steps linked to EOPs, EPHs or the operator di‐
alogs, the entries are taken from the basic automation;
for a library reference from the library operation. New
materials can also be created. For each output material
configured, the material, quality, scaling function and
required quantity are specified.
Either concrete values (internal formula) or reference to
values of an external formula.
Parameter Parameter list For the steps linked to EOPs, EPHs or the operator di‐
alogs, the parameters are taken from the basic automa‐
tion; for a library reference from the library operation.
New parameters of the type real, bool, integer, string
and enumeration can also be created.
Either concrete values (internal formula) or reference to
values of an external formula.
Process tags Measured variables
that will be logged
Measured variables for the batch report:
You can select measured variables for archiving. As
measured variables, the process values of the parame‐
ter blocks (EPAR_) linked with the EOP, EPH and
TAG_COLL blocks are available.
Change log List of modifications List in which the modifications made to the recipe are
documented.
ESIG Electronic signature Specifying electronic signatures in compliance with
FDA or 21 CFR Part 11:
The "electronic signatures" function allows the operator
to enter one or more signatures in the form of dialogs
similar to the logon prompts normal in Windows when
operator input is made to recipes, recipe steps etc.
BATCH Recipe Editor
10.4 Creating recipes
SIMATIC BATCH V8.2
578 Operating Manual, 02/2016, A5E35958174-AA

 Loading...
Loading...











