GEDRAFT LOGIQ P9/P7
D
IRECTION 5604324, REVISION 11 DRAFT (JANUARY 24, 2019) SERVICE MANUAL
Chapter 4 - Functional Checks 4-6
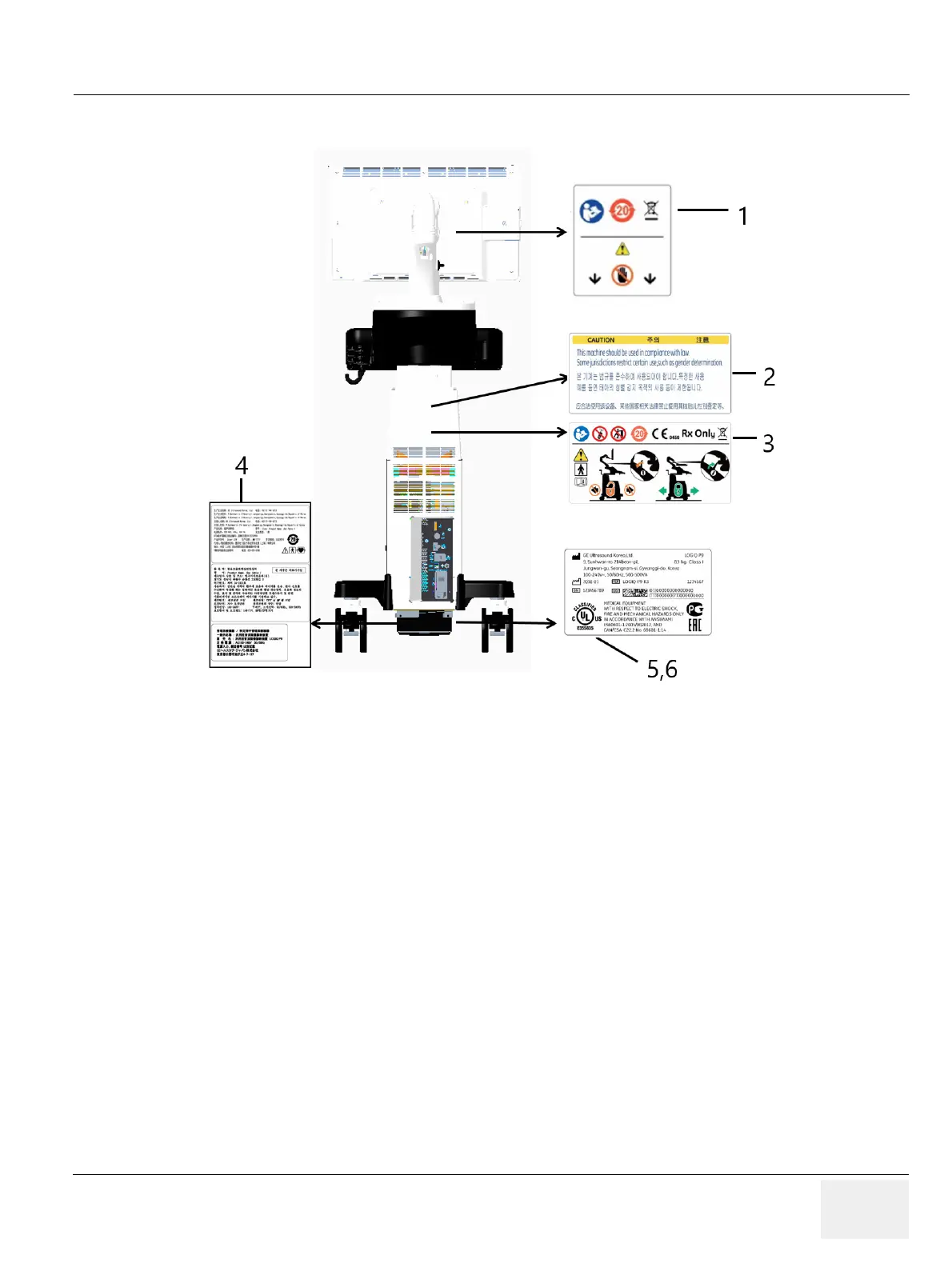
4-3-1-3 Appearance Inspection (R3)
* Required for Asia.
1) Check presence of LCD Caution Label.
2) Check presence of Gender Caution Label.l
NOTE: Some countries may not have "gender determination" label.
3) Check presence of Multi Caution Label.
4) Check presence of Rating Plate.
NOTE: Rating Plate design slightly differ depending on Console destination. At minimum simply check Console
number, manufacturing date and serial Number.
5) Check presence of UL Label. (But R3 system have UL symbol in LOGIQ P9/P7 RATING LABEL)
Figure 4-3 Label Location (R3)
1. LCD CAUTION LABEL
2. GENDER CAUTION LABEL (ONLY FOR INDIA, CHINA, KOREA)
3. MULTI CAUTION LABEL
4.
LOGIQ P9/P7 RATING LABEL (FOR CHINA, FOR KOREA, FOR
JAPAN)
5.
LOGIQ P9/P7 RATING LABEL

 Loading...
Loading...