GE MEDICAL SYSTEMS PROPRIETARY TO GE
D
IRECTION 2294854-100, REVISION 3 LOGIQ™ 9 PROPRIETARY MANUAL
Chapter 8 Replacement Procedures 8-135
Section 8-35
Verify and Update Vital Product Data
8-35-1 Introduction
It is essential that Vital Product Data (VPD) is verified or updated after any hardware change to the Back
End or Front End Processors.
NOTE: Failure to properly program Vital Product Data could cause adverse affects on system
operation and image quality.
The items of concern are:
- Verify that the Board or BEP Vital Product Gata is programmed
- Program the new Board or BEP Vital Product data. (BEP VPD is stored on the FEP
backplane).
8-35-2 Collecting Vital Product Data
For the Back End Processor, remove the system left side cover and read the label on the front of the
processor. The Part Number is there. The Functional Revision is the PSP# (number) on the label. Get
the date for the BEP from this BEP rating plate. All other dates are taken from the Console rating plate.
If the VPD Read Utility (8-35-3-3 "Acquisition Diagnostics - Read EEPROM Data" on page 8-138) shows
no data for the Equalization Board (EQ), obtain that data by removing the system right side cover,
remove the FEP cover, remove the EQ Board and obtain the data indicated in Table 8-9.
Table 8-9 BEP VPD Data
Circuit Board Name Part Number
Functional Revision
PWA Letter
Fab Revision
PWB Number
Bar Code or
Serial Number
Date
YYYYMMDD
BEP
BEP Rating Plate PSP Revison
N/A
No Dash Allowed BEP Rating Plate
2 0 0 _ _ _ 01
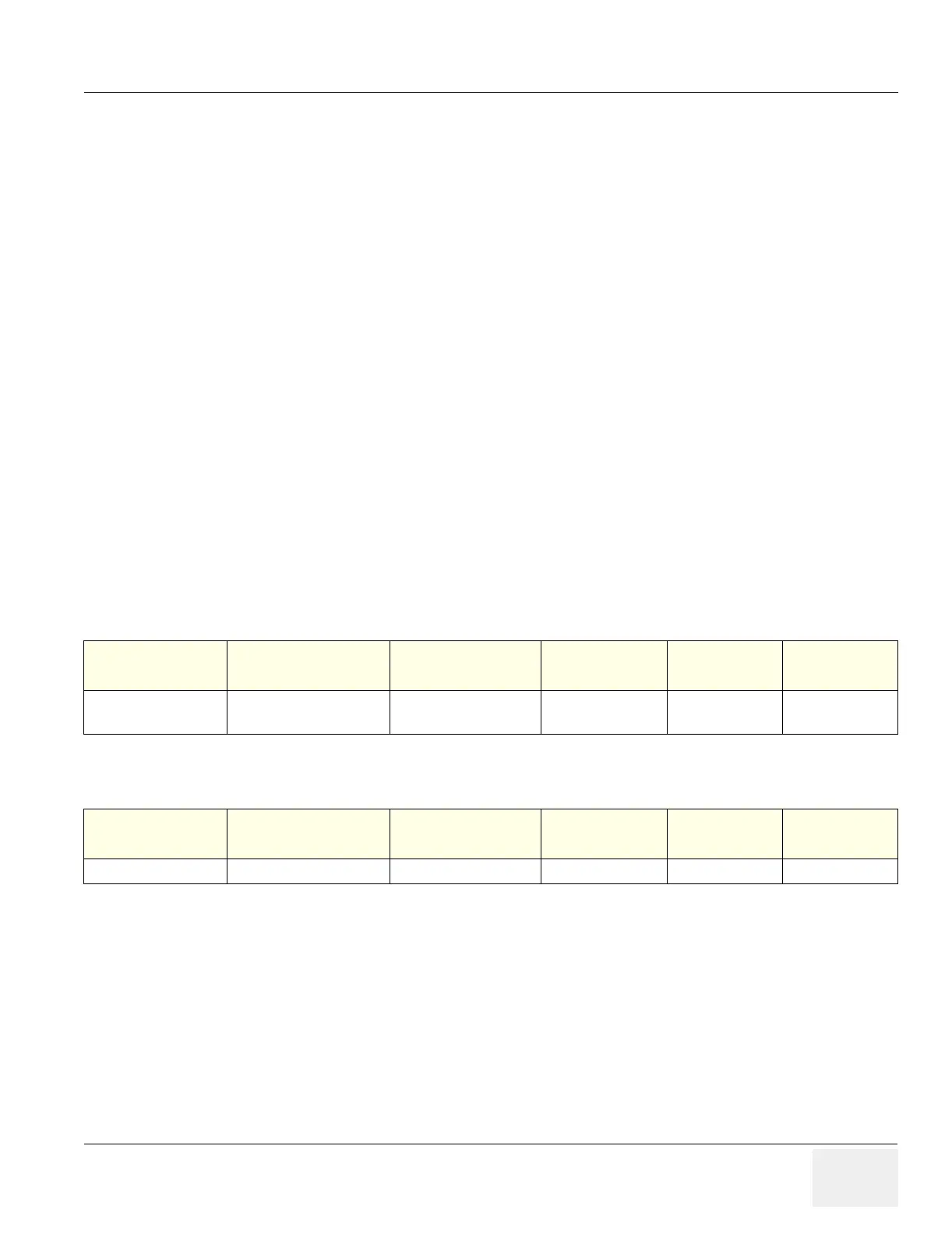
Table 8-10 Typical Circuit Board VPD Data
Circuit Board Name Part Number
Functional Revision
PWA Letter
Fab Revision
PWB Number
Bar Code or
Serial Number
Date
YYYYMMDD
Equalization 2260210 2 0 0 _ _ _ 01

 Loading...
Loading...